
Synopsis
Synopsis
0
CEP/COS
0
VMF
0
EDQM
0
USP
0
JP
0
Others
DRUG PRODUCT COMPOSITIONS
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Actimid
2. Cc 4047
3. Cc-4047
4. Cc4047
5. Imnovid
6. Pomalyst
1. 19171-19-8
2. Actimid
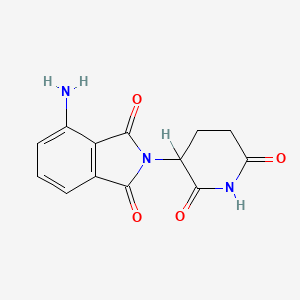
3. 4-amino-2-(2,6-dioxopiperidin-3-yl)isoindoline-1,3-dione
4. Cc-4047
5. Pomalyst
6. Imnovid
7. 4-aminothalidomide
8. 4-amino-2-(2,6-dioxo-3-piperidyl)isoindoline-1,3-dione
9. Imid 3
10. 3-amino-n-(2,6-dioxo-3-piperidyl)phthalimide
11. Cc 4047
12. 1h-isoindole-1,3(2h)-dione, 4-amino-2-(2,6-dioxo-3-piperidinyl)-
13. Imid-3
14. Pomalidomide (cc-4047)
15. 4-amino-2-(2,6-dioxopiperidin-3-yl)isoindole-1,3-dione
16. (s)-pomalidomide
17. D2ux06xlb5
18. Chebi:72690
19. Mfcd12756407
20. 1,3-dioxo-2-(2,6-dioxopiperidin-3-yl)-4-aminoisoindoline
21. 4-amino-2-(2,6-dioxo-3-piperidinyl)-1h-isoindole-1,3(2h)-dione
22. 4-amino-2-(2,6-dioxopiperidin-3-yl)-1h-isoindole-1,3(2h)-dione
23. 4-amino-2-(2,6-dioxopiperidin-3-yl)-2,3-dihydro-1h-isoindole-1,3-dione
24. Pomalidomide [usan:inn]
25. Unii-d2ux06xlb5
26. Hsdb 8222
27. Pomalyst (tn)
28. Cc4047
29. Pomalidomide- Bio-x
30. Imid1
31. Pomalidomide [mi]
32. Pomalidomide [inn]
33. Pomalidomide [jan]
34. 3-amino-n-(2,6-dioxo-3-piperidyl)phthalamide
35. Pomalidomide [usan]
36. 3-aminophthalimidoglutarimide
37. Pomalidomide [vandf]
38. Mls006011261
39. Chembl43452
40. Pomalidomide [who-dd]
41. Schembl369172
42. Gtpl7348
43. Pomalidomide (jan/usan/inn)
44. Pomalidomide [ema Epar]
45. 3-aminio-phthalimido-glutarimide
46. Schembl19250920
47. Bdbm65456
48. Imid-4047
49. Cdc-394
50. Dtxsid40893458
51. Pomalidomide, >=98% (hplc)
52. S-3-amino-phthalimido-glutarimide
53. Hms3655g05
54. Hms3744k07
55. Pomalidomide [orange Book]
56. Bcp02890
57. Bcp09107
58. Cfc83849
59. Am9718
60. Nsc767909
61. Nsc775351
62. S1567
63. Akos013400288
64. Ccg-264684
65. Cs-0165
66. Db08910
67. Nsc-767909
68. Nsc-775351
69. Sb16552
70. Ncgc00346551-01
71. Ncgc00346551-03
72. Ac-26970
73. As-17905
74. Bp-24477
75. Bp164278
76. Da-21486
77. Hy-10984
78. Smr004703012
79. Sy054807
80. Bcp0726000263
81. 3-(3-amino)-phtalamido-glutarimide
82. Ft-0697903
83. P2074
84. Sw218099-2
85. D08976
86. Ab01565777_02
87. 171p198
88. Sr-01000941573
89. J-012392
90. J-514302
91. Phthalimide, 3-amino-n-(2,6-dioxo-3-piperidyl)-
92. Q7227206
93. Sr-01000941573-1
94. 4-amino-2-(2,6-dioxo(3-piperidyl))isoindoline-1,3-dione
95. 4-amino-2-(2,6-dioxo-3-piperidinyl)isoindole-1,3-dione
96. 4-amino-2-(2,6-dioxo-3-piperidyl) Isoindoline -1,3-dione
97. 4-amino-2-((3rs)-2,6-dioxopiperidin-3-yl)-1h-isoindole-1,3(2h)-dione
98. Lipopolysaccharides From Escherichia Coli 055:b5 Pound>>lipopolysaccharides Pound>> Lipoglycans Pound>>endotoxins
| Molecular Weight | 273.24 g/mol |
|---|---|
| Molecular Formula | C13H11N3O4 |
| XLogP3 | 0.2 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 1 |
| Exact Mass | 273.07495584 g/mol |
| Monoisotopic Mass | 273.07495584 g/mol |
| Topological Polar Surface Area | 110 Ų |
| Heavy Atom Count | 20 |
| Formal Charge | 0 |
| Complexity | 504 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Pomalyst |
| PubMed Health | Pomalidomide (By mouth) |
| Drug Classes | Antineoplastic Agent |
| Active Ingredient | Pomalidomide |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 1mg; 4mg; 2mg; 3mg |
| Market Status | Prescription |
| Company | Celgene |
| 2 of 2 | |
|---|---|
| Drug Name | Pomalyst |
| PubMed Health | Pomalidomide (By mouth) |
| Drug Classes | Antineoplastic Agent |
| Active Ingredient | Pomalidomide |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 1mg; 4mg; 2mg; 3mg |
| Market Status | Prescription |
| Company | Celgene |
Angiogenesis Inhibitors; Immunologic Factors
National Library of Medicine's Medical Subject Headings. Linaclotide. Online file (MeSH, 2014). Available from, as of December 18, 2014: https://www.nlm.nih.gov/mesh/2014/mesh_browser/MBrowser.html
Pomalyst is indicated for patients with multiple myeloma who have received at least two prior therapies including lenalidomide and bortezomib and have demonstrated disease progression on or within 60 days of completion of the last therapy. Approval is based on response rate. Clinical benefit, such as improvement in survival or symptoms, has not been verified. /Included in US product label/
NIH; DailyMed. Current Medication Information for Pomalyst (Pomalidomide) Capsule (Revised: May 2014). Available from, as of December 31, 2014: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2b25ef01-5c9e-11e1-b86c-0800200c9a66
EXPL THER Pomalidomide /affected/ the regulation of fetal hemoglobin (HbF) making it a potential therapeutic agent for the treatment of non-malignant hematologic disorders such as sickle cell disease (SCD) and beta-thalassemia. In vitro pomalidomide was a more potent inducer of HbF than hydroxyurea (HU), the only treatment currently approved for SCD. Pomalidomide increased the expression of genes directing the production of HbF as well as gamma- and epsilon-globin gene transcription and expression during erythroid differentiation. In an in vivo knockout transgenic mouse model of SCD, pomalidomide (10 mg/kg; 5 QD/week x 8) stimulated erythropoiesis as indicated by bone marrow hyperplasia and increased extramedullary hematopoiesis, a trend toward higher reticulocytes and significantly higher red blood cell (RBC) levels. Pomalidomide significantly increased HbF expression with a trend toward higher gamma-globin chain A levels. The pomalidomide responder rate, defined as the percentage of animals that exceeded the maximum HbF and gamma-globin chain A levels in the vehicle group, reached 67% and 78% respectively. Among responders, pomalidomide induced a nearly 2-fold increase in HbF and the increase in gamma-globin chain A levels was significant and was similar to the approved HbF-inducing agent HU.
European Medicines Agency (EMA), Committee for Medicinal Products for Human Use (CHMP), European Public Assessment Report (EPAR): Pomalidomide Celgene (Pomalidomide) p.18 (2013). Available from, as of February 20, 2015: https://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002682/WC500147721.pdf
/BOXED WARNING/ WARNING: EMBRYO-FETAL TOXICITY. Embryo-Fetal Toxicity: Pomalyst is contraindicated in pregnancy. Pomalyst is a thalidomide analogue. Thalidomide is a known human teratogen that causes severe birth defects or embryo-fetal death. In females of reproductive potential, obtain 2 negative pregnancy tests before starting Pomalyst treatment. Females of reproductive potential must use 2 forms of contraception or continuously abstain from heterosexual sex during and for 4 weeks after stopping Pomalyst treatment. Pomalyst is only available through a restricted distribution program called Pomalyst REMS.
NIH; DailyMed. Current Medication Information for Pomalyst (Pomalidomide) Capsule (Revised: May 2014). Available from, as of December 31, 2014: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2b25ef01-5c9e-11e1-b86c-0800200c9a66
/BOXED WARNING/ WARNING: VENOUS THROMBOEMBOLISM. Deep venous thrombosis (DVT) and pulmonary embolism (PE) occur in patients with multiple myeloma treated with Pomalyst. Prophylactic anti-thrombotic measures were employed in the clinical trial. Consider prophylactic measures after assessing an individual patient's underlying risk factors
NIH; DailyMed. Current Medication Information for Pomalyst (Pomalidomide) Capsule (Revised: May 2014). Available from, as of February 27, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2b25ef01-5c9e-11e1-b86c-0800200c9a66
Use of pomalidomide should be avoided in patients with serum aminotransferase (ALT and AST) concentrations exceeding 3 times the upper limit of normal (ULN) and bilirubin concentrations exceeding 2 mg/dL. Use of pomalidomide also should be avoided in patients with serum creatinine concentrations exceeding 3 mg/dL. Safety and efficacy have not been established in these patients.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 1200
Pomalidomide may cause fetal toxicity; pomalidomide is a structural analog of thalidomide, a known human teratogen, and teratogenic and other fetotoxic effects of pomalidomide (e.g., musculoskeletal anomalies and deformities; absence of internal organs, including bladder and thyroid; defects of internal organ systems, including cardiovascular, respiratory, renal, hepatic, and CNS abnormalities; increased fetal resorptions) have been demonstrated in animals. Therefore, pomalidomide is contraindicated in women who are pregnant.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 1200
For more Drug Warnings (Complete) data for Pomalidomide (21 total), please visit the HSDB record page.
Pomalidomide is indicated for patients with multiple myeloma who have received at least two prior therapies including lenalidomide and bortezomib and have demonstrated disease progression on or within 60 days of completion of the last therapy.
FDA Label
Imnovid in combination with bortezomib and dexamethasone is indicated in the treatment of adult patients with multiple myeloma who have received at least one prior treatment regimen including lenalidomide.
Imnovid in combination with dexamethasone is indicated in the treatment of adult patients with relapsed and refractory multiple myeloma who have received at least two prior treatment regimens, including both lenalidomide and bortezomib, and have demonstrated disease progression on the last therapy.
Treatment of post-essential thrombocythaemia myelofibrosis, Treatment of post-polycythaemia vera myelofibrosis
Pomalidomide is more potent than thalidomide (100-times) and lenalidomide (10-times).
Angiogenesis Inhibitors
Agents and endogenous substances that antagonize or inhibit the development of new blood vessels. (See all compounds classified as Angiogenesis Inhibitors.)
Immunologic Factors
Biologically active substances whose activities affect or play a role in the functioning of the immune system. (See all compounds classified as Immunologic Factors.)
L04AX06
L04AX06
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
L - Antineoplastic and immunomodulating agents
L04 - Immunosuppressants
L04A - Immunosuppressants
L04AX - Other immunosuppressants
L04AX06 - Pomalidomide
Absorption
Pomalidomide is generally well absorbed. The major circulating component is the parent compound. Tmax, single oral dose = 2 -3 hours. When 4 mg of promalidomide is given to patients with multiple myeloma, the steady-state pharmacokinetic parameters are as follows: AUC(T) = 400 ng.hr/mL; Cmax = 75 ng/mL. Promalidomide accumulates following multiple doses.
Route of Elimination
When a single oral dose (2mg) is given to healthy subjects, 73% of the dose was eliminated in urine. 15% of the dose was eliminated in feces. 2% and 8% of the dose eliminated unchanged as pomalidomide in urine and feces, respectively.
Volume of Distribution
Mean apparent volume of distribution (Vd/F), steady-state = 62 - 138 L
Clearance
Total body clearance = 7-10 L/hour
Pomalidomide has a mean apparent volume of distribution (Vd/F) between 62 and 138 L at steady state. Pomalidomide is distributed in semen of healthy subjects at a concentration of approximately 67% of plasma level at 4 hours post-dose (approximate Tmax) after 4 days of once-daily dosing at 2 mg. Human plasma protein binding ranges from 12% to 44% and is not concentration dependent. Pomalidomide is a substrate for P-glycoprotein (P-gp).
NIH; DailyMed. Current Medication Information for Pomalyst (Pomalidomide) Capsule (Revised: May 2014). Available from, as of February 13, 2014: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2b25ef01-5c9e-11e1-b86c-0800200c9a66
In patients with multiple myeloma who received Pomalyst 4 mg daily alone or in combination with dexamethasone, pomalidomide steady-state drug exposure was characterized by AUC(T) of 400 ng*h/mL and Cmax of 75 ng/mL. Following multiple doses, pomalidomide has an accumulation ratio of 27% to 31%.
NIH; DailyMed. Current Medication Information for Pomalyst (Pomalidomide) Capsule (Revised: May 2014). Available from, as of February 13, 2014: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2b25ef01-5c9e-11e1-b86c-0800200c9a66
Following a single oral administration of (14)C-pomalidomide (2 mg) to healthy subjects, approximately 73% and 15% of the radioactive dose was eliminated in urine and feces, respectively, with approximately 2% and 8% of the radiolabeled dose eliminated unchanged as pomalidomide in urine and feces.
NIH; DailyMed. Current Medication Information for Pomalyst (Pomalidomide) Capsule (Revised: May 2014). Available from, as of February 13, 2014: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2b25ef01-5c9e-11e1-b86c-0800200c9a66
Pomalidomide has a mean total body clearance (CL/F) of 7-10 L/hr.
NIH; DailyMed. Current Medication Information for Pomalyst (Pomalidomide) Capsule (Revised: May 2014). Available from, as of February 13, 2014: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2b25ef01-5c9e-11e1-b86c-0800200c9a66
For more Absorption, Distribution and Excretion (Complete) data for Pomalidomide (12 total), please visit the HSDB record page.
Promalidomide is hepatically metabolized by CYP1A2 and CYP3A4. The metabolites are 26-fold less active than the parent compound. Minor contributions from CYP2C19 and CYP2D6 have been observed in vitro.
In hepatocytes from rabbit and human, and in vivo in rat, monkey and human, pomalidomide was metabolized primarily via hydroxylation of the phthalimide ring (M14, M16 and M17) followed by glucuronidation (M12 and M13), hydrolysis of the glutarimide ring (M10 and M11), and hydrolysis of the phthalimide ring (M2). There were no unique or disproportionate metabolites observed in humans, compared to rats and monkeys.
European Medicines Agency (EMA), Committee for Medicinal Products for Human Use (CHMP), European Public Assessment Report (EPAR): Pomalidomide Celgene (Pomalidomide) p.20 (2013). Available from, as of February 20, 2015: https://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002682/WC500147721.pdf
Pomalidomide is primarily metabolized in the liver by CYP1A2 and CYP3A4. In vitro, CYP1A2 and CYP3A4 were identified as the primary enzymes involved in the CYP-mediated hydroxylation of pomalidomide, with additional minor contributions from CYP2C19 and CYP2D6.
NIH; DailyMed. Current Medication Information for Pomalyst (Pomalidomide) Capsule (Revised: May 2014). Available from, as of February 13, 2014: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2b25ef01-5c9e-11e1-b86c-0800200c9a66
Healthy subjects = 9.4 hours; Multiple myeloma patients = 7.5 hours.
... the terminal half-lives of pomalidomide in animals ranged from mean values of 4 to 7 hours following an IV dose.
European Medicines Agency (EMA), Committee for Medicinal Products for Human Use (CHMP), European Public Assessment Report (EPAR): Pomalidomide Celgene (Pomalidomide) p.19 (2013). Available from, as of February 20, 2015: https://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002682/WC500147721.pdf
Pomalidomide is eliminated with a median plasma half-life of approximately 9.5 hours in healthy subjects and approximately 7.5 hours in patients with multiple myeloma.
NIH; DailyMed. Current Medication Information for Pomalyst (Pomalidomide) Capsule (Revised: May 2014). Available from, as of February 13, 2014: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2b25ef01-5c9e-11e1-b86c-0800200c9a66
Promalidomide is an immunomodulatory agent with antineoplastic activity. It is shown to inhibit the proliferation and induce apoptosis of various tumour cells. Furthermore, promalidomide enhances T cell and natural killer (NK) cell-mediated immunity and inhibited the production of pro-inflammatory cytokines, like TNF-alpha or IL-6, by monocytes. The primary target of promalidomide is thought to be the protein cereblon. It binds to this target and inhibits ubiquitin ligase activity. It is also a transcriptional inhibitor of COX2.
In vitro studies have identified a molecular mechanism for the pleiotropic effects of pomalidomide in multiple myeloma (MM) and in T cells. Specifically, pomalidomide bound to the protein cereblon (CRBN), part of an E3 ligase complex, and the expression levels of CRBN in myeloma cells was linked to both the efficacy of pomalidomide and to the acquisition of resistance to lenalidomide. Pomalidomide was claimed to have direct antiproliferative activity against B cell lines derived from MM and Burkitt's lymphoma patients. Pomalidomide in combination with dexamethasone increased this effect in a dose dependent manner.
European Medicines Agency (EMA), Committee for Medicinal Products for Human Use (CHMP), European Public Assessment Report (EPAR): Pomalidomide Celgene (Pomalidomide) p.16 (2013). Available from, as of February 20, 2015: https://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002682/WC500147721.pdf
Although several mechanisms have been proposed to explain the activity of thalidomide, lenalidomide and pomalidomide in multiple myeloma (MM), including demonstrable anti-angiogenic, anti-proliferative and immunomodulatory effects, the precise cellular targets and molecular mechanisms have only recently become clear. A landmark study recently identified cereblon (CRBN) as a primary target of thalidomide teratogenicity. Subsequently it was demonstrated that CRBN is also required for the anti-myeloma activity of thalidomide and related drugs, the so-called immune-modulatory drugs (IMiDs). Low CRBN expression was found to correlate with drug resistance in MM cell lines and primary MM cells. One of the downstream targets of CRBN identified is interferon regulatory factor 4 (IRF4), which is critical for myeloma cell survival and is down-regulated by IMiD treatment. CRBN is also implicated in several effects of IMiDs, such as down-regulation of tumor necrosis factor-alpha (TNF-a) and T cell immunomodulatory activity, demonstrating that the pleotropic actions of the IMiDs are initiated by binding to CRBN. Future dissection of CRBN downstream signaling will help to delineate the underlying mechanisms for IMiD action and eventually lead to development of new drugs with more specific anti-myeloma activities. It may also provide a biomarker to predict IMiD response and resistance.
PMID:22966948 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3931443 Zhu YX et al; Leuk Lymphoma 54 (4): 683-7 (2013)
The Cul4-Rbx1-DDB1-Cereblon E3 ubiquitin ligase complex is the target of thalidomide, lenalidomide and pomalidomide, therapeutically important drugs for multiple myeloma and other B-cell malignancies. These drugs directly bind Cereblon (CRBN) and promote the recruitment of substrates Ikaros (IKZF1) and Aiolos (IKZF3) to the E3 complex, thus leading to substrate ubiquitination and degradation. Here we present the crystal structure of human CRBN bound to DDB1 and the drug lenalidomide. A hydrophobic pocket in the thalidomide-binding domain (TBD) of CRBN accommodates the glutarimide moiety of lenalidomide, whereas the isoindolinone ring is exposed to solvent. We also solved the structures of the mouse TBD in the apo state and with thalidomide or pomalidomide. Site-directed mutagenesis in lentiviral-expression myeloma models showed that key drug-binding residues are critical for antiproliferative effects.
PMID:25108355 Chamberlain PP et al; Nat Struct Mol Biol 21 (9): 803-9 (2014)
In the 1950s, the drug thalidomide, administered as a sedative to pregnant women, led to the birth of thousands of children with multiple defects. Despite the teratogenicity of thalidomide and its derivatives lenalidomide and pomalidomide, these immunomodulatory drugs (IMiDs) recently emerged as effective treatments for multiple myeloma and 5q-deletion-associated dysplasia. IMiDs target the E3 ubiquitin ligase CUL4-RBX1-DDB1-CRBN (known as CRL4(CRBN)) and promote the ubiquitination of the IKAROS family transcription factors IKZF1 and IKZF3 by CRL4(CRBN). Here we present crystal structures of the DDB1-CRBN complex bound to thalidomide, lenalidomide and pomalidomide. The structure establishes that CRBN is a substrate receptor within CRL4(CRBN) and enantioselectively binds IMiDs. Using an unbiased screen, we identified the homeobox transcription factor MEIS2 as an endogenous substrate of CRL4(CRBN). Our studies suggest that IMiDs block endogenous substrates (MEIS2) from binding to CRL4(CRBN) while the ligase complex is recruiting IKZF1 or IKZF3 for degradation. This dual activity implies that small molecules can modulate an E3 ubiquitin ligase and thereby upregulate or downregulate the ubiquitination of proteins.
PMID:25043012 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4423819 Fischer ES et al; Nature 512 (7512): 49-53 (2014)

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
ABOUT THIS PAGE
76
PharmaCompass offers a list of Pomalidomide API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Pomalidomide manufacturer or Pomalidomide supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Pomalidomide manufacturer or Pomalidomide supplier.
PharmaCompass also assists you with knowing the Pomalidomide API Price utilized in the formulation of products. Pomalidomide API Price is not always fixed or binding as the Pomalidomide Price is obtained through a variety of data sources. The Pomalidomide Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Pomalyst manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Pomalyst, including repackagers and relabelers. The FDA regulates Pomalyst manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Pomalyst API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Pomalyst manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Pomalyst supplier is an individual or a company that provides Pomalyst active pharmaceutical ingredient (API) or Pomalyst finished formulations upon request. The Pomalyst suppliers may include Pomalyst API manufacturers, exporters, distributors and traders.
click here to find a list of Pomalyst suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Pomalyst DMF (Drug Master File) is a document detailing the whole manufacturing process of Pomalyst active pharmaceutical ingredient (API) in detail. Different forms of Pomalyst DMFs exist exist since differing nations have different regulations, such as Pomalyst USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Pomalyst DMF submitted to regulatory agencies in the US is known as a USDMF. Pomalyst USDMF includes data on Pomalyst's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Pomalyst USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Pomalyst suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Pomalyst Drug Master File in Japan (Pomalyst JDMF) empowers Pomalyst API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Pomalyst JDMF during the approval evaluation for pharmaceutical products. At the time of Pomalyst JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Pomalyst suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Pomalyst Drug Master File in Korea (Pomalyst KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Pomalyst. The MFDS reviews the Pomalyst KDMF as part of the drug registration process and uses the information provided in the Pomalyst KDMF to evaluate the safety and efficacy of the drug.
After submitting a Pomalyst KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Pomalyst API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Pomalyst suppliers with KDMF on PharmaCompass.
A Pomalyst written confirmation (Pomalyst WC) is an official document issued by a regulatory agency to a Pomalyst manufacturer, verifying that the manufacturing facility of a Pomalyst active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Pomalyst APIs or Pomalyst finished pharmaceutical products to another nation, regulatory agencies frequently require a Pomalyst WC (written confirmation) as part of the regulatory process.
click here to find a list of Pomalyst suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Pomalyst as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Pomalyst API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Pomalyst as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Pomalyst and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Pomalyst NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Pomalyst suppliers with NDC on PharmaCompass.
Pomalyst Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Pomalyst GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Pomalyst GMP manufacturer or Pomalyst GMP API supplier for your needs.
A Pomalyst CoA (Certificate of Analysis) is a formal document that attests to Pomalyst's compliance with Pomalyst specifications and serves as a tool for batch-level quality control.
Pomalyst CoA mostly includes findings from lab analyses of a specific batch. For each Pomalyst CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Pomalyst may be tested according to a variety of international standards, such as European Pharmacopoeia (Pomalyst EP), Pomalyst JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Pomalyst USP).

