
Synopsis
0
USDMF
0
CEP/COS
0
KDMF
0
NDC API
0
VMF
0
FDA Orange Book
0
Canada
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
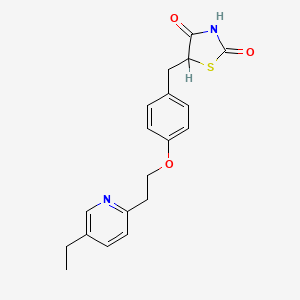

1. 5-(4-(2-(5-ethyl-2-pyridyl)ethoxy)benzyl)-2,4-thiazolidinedione
2. Actos
3. Ad 4833
4. Ad-4833
5. Ad4833
6. Pioglitazone Hydrochloride
7. U 72107a
8. U-72107a
9. U72,107a
10. U72107a
1. 111025-46-8
2. Actos
3. Glustin
4. Zactos
5. 105355-27-9
6. Pioglitazona
7. Pioglitazonum
8. Pioglitazonum [inn-latin]
9. 5-(4-(2-(5-ethylpyridin-2-yl)ethoxy)benzyl)thiazolidine-2,4-dione
10. Pioglitazona [inn-spanish]
11. Duetact
12. Ad-4833
13. U 72107
14. Pioglitazone (inn)
15. 105390-47-4
16. 5-[[4-[2-(5-ethylpyridin-2-yl)ethoxy]phenyl]methyl]-1,3-thiazolidine-2,4-dione
17. Chebi:8228
18. Pioglitazone (actos)
19. 5-[[4-[2-(5-ethyl-2-pyridinyl)ethoxy]phenyl]methyl]-2,4-thiazolidinedione
20. U-72107
21. 5-[4-[2-(5-ethyl-2-pyridyl)ethoxy]benzyl]thiazolidine-2,4-dione
22. Actos (tn)
23. 5-{4-[2-(5-ethylpyridin-2-yl)ethoxy]benzyl}-1,3-thiazolidine-2,4-dione
24. 2,4-thiazolidinedione, 5-[[4-[2-(5-ethyl-2-pyridinyl)ethoxy]phenyl]methyl]-
25. X4ov71u42s
26. 5-({4-[2-(5-ethylpyridin-2-yl)ethoxy]phenyl}methyl)-1,3-thiazolidine-2,4-dione
27. Mfcd00865504
28. 5-[4-[2-(5-ethyl-2-pyridyl)ethoxy]benzyl]-2,4-thiazolidinedione
29. Pioglitazone [inn]
30. 5-((4-(2-(5-ethyl-2-pyridinyl)ethoxy)phenyl)methyl)-2,4-thiazolidinedione
31. Pioglitazone [ban:inn]
32. Pioglitazone [inn:ban]
33. Piozone
34. Pioglu
35. 2,4-thiazolidinedione, 5-((4-(2-(5-ethyl-2-pyridinyl)ethoxy)phenyl)methyl)-
36. [( Inverted Exclamation Marka)-5-[[4-[2-(5-ethyl-2-pyridinyl) Ethoxy] Phenyl] Methyl]-2,4-] Thiazolidinedione Monohydrochlorid
37. Hsdb 7322
38. Drx-065
39. Unii-x4ov71u42s
40. Sr-01000763737
41. 5-(4-(2-(5-ethyl-2-pyridyl)ethoxy)benzyl)thiazolidine-2,4-dione
42. 2,4-thiazolidinedione, 5-[[4-[2-(5-ethyl-2-pyridinyl)ethoxy]phenyl]methyl]-, (+/-)-
43. 2,4-thiazolidinedione, 5-((4-(2-(5-ethyl-2-pyridinyl)ethoxy)phenyl)methyl)-, (+/-)-
44. Pioglitazone-[d4]
45. Pioglitazone- Bio-x
46. 5-{4-[2-(5-ethyl-2-pyridyl)ethoxy]benzyl}-2,4-thiazolidinedione
47. Hs-0047
48. Spectrum_001623
49. Spectrum2_001679
50. Spectrum3_001002
51. Spectrum4_001130
52. Spectrum5_001480
53. Spectrum5_002067
54. Pioglitazone [mi]
55. Pioglitazone [hsdb]
56. Pioglitazone [iarc]
57. Schembl4121
58. Pioglitazone [vandf]
59. Bspbio_002723
60. Kbiogr_001619
61. Kbioss_002103
62. Mls006011848
63. Pioglitazone [who-dd]
64. Spbio_001897
65. Gtpl2694
66. Pioglitazone [ema Epar]
67. Dtxsid3037129
68. Kbio2_002103
69. Kbio2_004671
70. Kbio2_007239
71. Kbio3_001943
72. Hms2089h14
73. Hms3651d09
74. Hms3712e16
75. Hms3884l10
76. Pharmakon1600-01504401
77. Act02635
78. Bcp26474
79. Bbl029068
80. Bdbm50103521
81. Nsc758876
82. S2590
83. Stl309607
84. Stl373406
85. (+/-)-5-[p-[2-(ethyl-2-pyridyl)ethoxy]benzyl]-2,4-thiazolidinedione
86. Akos015894953
87. Akos022109420
88. Ac-1021
89. Ccg-220107
90. Cs-1700
91. Db01132
92. Sb17323
93. (+/-)-5-[[4-[2-(5-ethyl-2-pyridinyl)-ethoxy]phenyl]methyl]-2,4-thiazolidinedione
94. 2,4-thiazolidinedione, 5-((4-(2-(5-ethyl-2-pyridinyl)ethoxy)phenyl)methyl)-, (+-)-
95. Ncgc00163128-01
96. Ncgc00163128-02
97. Ncgc00163128-03
98. Ncgc00163128-04
99. Ncgc00163128-05
100. Ncgc00163128-06
101. Ncgc00163128-07
102. Bp164273
103. Hy-13956
104. Smr002204015
105. Sy017473
106. Sbi-0206791.p001
107. Db-027350
108. Ft-0601906
109. Ft-0645030
110. Sw197561-3
111. C07675
112. D08378
113. Ab00698454-10
114. Ab00698454_11
115. Ab00698454_12
116. Ab00698454_13
117. 355p279
118. A802277
119. Q417765
120. J-002506
121. J-516181
122. Sr-01000763737-5
123. Brd-a48430263-003-02-4
124. Brd-a48430263-003-06-5
125. 5-[4-[2-(5-ethyl-2-pyridyl) Ethoxy]benzyl]-2,4-thiazolidinedione
126. 5-[4-[2-(5-ethyl-2-pyridyl)eth-oxy]benzyl]-2,4-thiazolidinedione
127. 5-{4-[2-(5-ethyl-2-pyridyl)ethoxy]benzyl}-2,4thiazolidinedione
128. 5-(4-(2-(5-ethylpyridin-2-yl)ethoxy)benzyl)-thiazolidine-2,4-dione
129. 5-[[4-[2-(5-ethyl-2-pyridinyl)ethoxy]phenyl]methyl-2,4-thiazolidinedione
130. 5-[[4-[2-(5-ethyl-2-pyridyl)ethoxy] Phenyl]methyl]-2,4-thiazolidinedione
131. (+/-)-5-((4-(2-(5-ethyl-2-pyridinyl)ethoxy)phenyl)methyl)-2,4-thiazolidinedione
132. (+/-)-5-(p-(2-(5-ethyl-2-pyridyl)ethoxy)benzyl)-2,4-thiazolidinedione
133. (5s)-5-[[4-[2-(5-ethyl-2-pyridyl)ethoxy]phenyl]methyl]thiazolidine-2,4-dione;pioglitazone
134. (rs)-5-(4-(2-(5-ethylpyridin-2-yl)ethoxy)benzyl)thiazolidine-2,4-dione
135. 2,4-thiazolidinedione, 5-[[4-[2-(5-ethyl-2-pyridinyl)ethoxy]phenyl]methyl]- (9ci)
136. 5-[[4-[2-[(5-ethyl-2-pyridyl)]ethoxy]phenyl]methyl]thiazolidine- 2,4-dione
137. 5-{4-[2-(5-ethylpyridin-2-yl)ethoxy]benzyl}-4-hydroxy-1,3-thiazol-2(5h)-one
138. 198077-89-3
| Molecular Weight | 356.4 g/mol |
|---|---|
| Molecular Formula | C19H20N2O3S |
| XLogP3 | 3.8 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 7 |
| Exact Mass | 356.11946368 g/mol |
| Monoisotopic Mass | 356.11946368 g/mol |
| Topological Polar Surface Area | 93.6 Ų |
| Heavy Atom Count | 25 |
| Formal Charge | 0 |
| Complexity | 466 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 6 | |
|---|---|
| Drug Name | Actos |
| PubMed Health | Pioglitazone (By mouth) |
| Drug Classes | Antidiabetic |
| Drug Label | ACTOS (pioglitazone hydrochloride) is an oral antidiabetic agent that acts primarily by decreasing insulin resistance. ACTOS is used in the management of type2 diabetes mellitus (also known as non-insulin-dependent diabetes mellitus [NIDDM] or adul... |
| Active Ingredient | Pioglitazone hydrochloride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 15mg base; eq 30mg base; eq 45mg base |
| Market Status | Prescription |
| Company | Takeda Pharms Usa |
| 2 of 6 | |
|---|---|
| Drug Name | Duetact |
| PubMed Health | Pioglitazone/Glimepiride (By mouth) |
| Drug Classes | Antidiabetic |
| Active Ingredient | pioglitazone hydrochloride; Glimepiride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 4mg; 2mg; 30mg |
| Market Status | Prescription |
| Company | Takeda Pharms Usa |
| 3 of 6 | |
|---|---|
| Drug Name | Pioglitazone |
| PubMed Health | Pioglitazone (By mouth) |
| Drug Classes | Antidiabetic |
| Drug Label | ACTOS (pioglitazone hydrochloride) is an oral antidiabetic agent that acts primarily by decreasing insulin resistance. ACTOS is used in the management of type2 diabetes mellitus (also known as non-insulin-dependent diabetes mellitus [NIDDM] or adul... |
| Active Ingredient | Pioglitazone hydrochloride |
| Dosage Form | Tablet |
| Route | oral |
| Strength | 30mg; 15mg; 45mg |
| Market Status | Tentative Approval |
| Company | Alphapharm |
| 4 of 6 | |
|---|---|
| Drug Name | Actos |
| PubMed Health | Pioglitazone (By mouth) |
| Drug Classes | Antidiabetic |
| Drug Label | ACTOS (pioglitazone hydrochloride) is an oral antidiabetic agent that acts primarily by decreasing insulin resistance. ACTOS is used in the management of type2 diabetes mellitus (also known as non-insulin-dependent diabetes mellitus [NIDDM] or adul... |
| Active Ingredient | Pioglitazone hydrochloride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 15mg base; eq 30mg base; eq 45mg base |
| Market Status | Prescription |
| Company | Takeda Pharms Usa |
| 5 of 6 | |
|---|---|
| Drug Name | Duetact |
| PubMed Health | Pioglitazone/Glimepiride (By mouth) |
| Drug Classes | Antidiabetic |
| Active Ingredient | pioglitazone hydrochloride; Glimepiride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 4mg; 2mg; 30mg |
| Market Status | Prescription |
| Company | Takeda Pharms Usa |
| 6 of 6 | |
|---|---|
| Drug Name | Pioglitazone |
| PubMed Health | Pioglitazone (By mouth) |
| Drug Classes | Antidiabetic |
| Drug Label | ACTOS (pioglitazone hydrochloride) is an oral antidiabetic agent that acts primarily by decreasing insulin resistance. ACTOS is used in the management of type2 diabetes mellitus (also known as non-insulin-dependent diabetes mellitus [NIDDM] or adul... |
| Active Ingredient | Pioglitazone hydrochloride |
| Dosage Form | Tablet |
| Route | oral |
| Strength | 30mg; 15mg; 45mg |
| Market Status | Tentative Approval |
| Company | Alphapharm |
Hypoglycemic Agents
National Library of Medicine's Medical Subject Headings. Pioglitazone. Online file (MeSH, 2018). Available from, as of January 22, 2018: https://meshb.nlm.nih.gov/search
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Pioglitazone is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of January 22, 2018: https://clinicaltrials.gov/
Pioglitazone is used alone (monotherapy) or in combination with a sulfonylurea antidiabetic agent, metformin (either as a fixed-combination preparation or as individual drugs given concurrently), or insulin as an adjunct to diet and exercise for the management of type 2 diabetes mellitus. Pioglitazone is used also in fixed combination with glimepiride in patients with type 2 diabetes mellitus who are already receiving pioglitazone and a sulfonylurea separately or who are inadequately controlled on a sulfonylurea or pioglitazone alone. In patients whose hyperglycemia cannot be controlled with these other antidiabetic agents, pioglitazone should be added to, not substituted for, such antidiabetic therapy. /Included in US product labeling/
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 3297
/EXPL THER/ Peroxisome proliferator activated receptor gamma-activating drugs show various salutary effects in preclinical models of neurodegenerative disease. The decade-long clinical usage of these drugs as antidiabetics now allows for evaluation of patient-oriented data sources. Using observational data from 2004-2010, we analyzed the association of pioglitazone and incidence of dementia in a prospective cohort study of 145,928 subjects aged >/= 60 years who, at baseline, were free of dementia and insulin-dependent diabetes mellitus. We distinguished between nondiabetics, diabetics without pioglitazone, diabetics with prescriptions of <8 calendar quarters of pioglitazone, and diabetics with =8 quarters. Cox proportional hazard models explored the relative risk (RR) of dementia incidence dependent on pioglitazone use adjusted for sex, age, use of rosiglitazone or metformin, and cardiovascular comorbidities. Long-term use of pioglitazone was associated with a lower dementia incidence. Relative to nondiabetics, the cumulative long-term use of pioglitazone reduced the dementia risk by 47% (RR=0.53, p=0.029). If diabetes patients used pioglitazone <8 quarters, the dementia risk was comparable to those of nondiabetics (RR=1.16, p=0.317), and diabetes patients without a pioglitazone treatment had a 23% increase in dementia risk (RR=1.23, p<0.001). We did not find evidence for age effects, nor for selection into pioglitazone treatment due to obesity. These findings indicate that pioglitazone treatment is associated with a reduced dementia risk in initially non-insulin-dependent diabetes mellitus patients. Prospective clinical trials are needed to evaluate a possible neuroprotective effect in these patients in an ageing population.
PMID:25974006 Heneka MT et al; Ann Neurol 78 (2): 284-94 (2015)
For more Therapeutic Uses (Complete) data for Pioglitazone (9 total), please visit the HSDB record page.
/BOXED WARNING/ WARNING: CONGESTIVE HEART FAILURE. Thiazolidinediones, including pioglitazone hydrochloride, cause or exacerbate congestive heart failure in some patients. After initiation of pioglitazone tablets, and after dose increases, monitor patients carefully for signs and symptoms of heart failure (e.g., excessive, rapid weight gain, dyspnea, and/or edema). If heart failure develops, it should be managed according to current standards of care and discontinuation or dose reduction of pioglitazone hydrochloride must be considered. Pioglitazone tablets are not recommended in patients with symptomatic heart failure. Initiation of pioglitazone hydrochloride in patients with established NYHA Class III or IV heart failure is contraindicated.
NIH; DailyMed. Current Medication Information for Pioglitazone Hydrochloride Tablet (Updated: December 27, 2017). Available from, as of January 26, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=8f2d7000-37ca-4e09-98ec-07d1c0354cb3
Thiazolidinediones, including pioglitazone, alone or in combination with other antidiabetic agents, can cause fluid retention, which may lead to or exacerbate congestive heart failure (CHF). Use of thiazolidinediones is associated with an approximately twofold increased risk of CHF. Use of pioglitazone in combination with insulin or in patients with New York Heart Association (NYHA) class I or II heart failure may increase the risk. Patients should be observed for signs and symptoms of CHF (e.g., dyspnea, rapid weight gain, edema, unexplained cough or fatigue), especially during initiation of therapy and dosage titration. If signs and symptoms of CHF develop, the disorder should be managed according to current standards of care. In addition, a decrease in the dosage or discontinuance of pioglitazone must be considered in such patients.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 3298
Patients with New York Heart Association (NYHA) class III or IV cardiac status with or without congestive heart failure (CHF) or with an acute coronary event were not studied in clinical trials of pioglitazone; initiation of therapy with the drug is contraindicated in patients with NYHA class III or IV heart failure. Use of pioglitazone is not recommended in patients with symptomatic heart failure. Caution should be exercised in patients with edema and in those who are at risk for CHF. Thiazolidinedione therapy should not be initiated in hospitalized patients with diabetes mellitus because of the delayed onset of action and because possible drug-related increases in vascular volume and CHF may complicate care of patients with hemodynamic changes induced by coexisting conditions or in-hospital interventions.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 3298-9
Risk for pregnancy unless contraceptive measures initiated; anovulatory premenopausal women with insulin resistance may resume ovulation during therapy. The frequency of resumption of ovulation with pioglitazone therapy has not been evaluated in clinical studies, and, therefore, is unknown. If menstrual dysfunction occurs, weigh risks versus benefits of continued pioglitazone.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 3299
For more Drug Warnings (Complete) data for Pioglitazone (20 total), please visit the HSDB record page.
Pioglitazone is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. It is also available in combination with [metformin], [glimepiride], or [alogliptin] for the same indication.
FDA Label
Pioglitazone is indicated as second or third line treatment of type-2 diabetes mellitus as described below:
- as
* monotherapy: :
- in adult patients (particularly overweight patients) inadequately controlled by diet and exercise for whom metformin is inappropriate because of contraindications or intolerance.
- as
* dual oral therapy: in combination with:
- metformin, in adult patients (particularly overweight patients) with insufficient glycaemic control despite maximal tolerated dose of monotherapy with metformin;
- a sulphonylurea, only in adult patients who show intolerance to metformin or for whom metformin is contraindicated, with insufficient glycaemic control despite maximal tolerated dose of monotherapy with a sulphonylurea;
- as
* triple oral therapy: in combination with:
- metformin and a sulphonylurea, in adult patients (particularly overweight patients) with insufficient glycaemic control despite dual oral therapy.
Pioglitazone is also indicated for combination with insulin in type-2 diabetes mellitus adult patients with insufficient glycaemic control on insulin for whom metformin is inappropriate because of contraindications or intolerance.
After initiation of therapy with pioglitazone, patients should be reviewed after three to six months to assess adequacy of response to treatment (e. g. reduction in HbA1c). In patients who fail to show an adequate response, pioglitazone should be discontinued. In light of potential risks with prolonged therapy, prescribers should confirm at subsequent routine reviews that the benefit of pioglitazone is maintained.
Pioglitazone is indicated in the treatment of type-2 diabetes mellitus as
* monotherapy: :
- in adult patients (particularly overweight patients) inadequately controlled by diet and exercise for whom metformin is inappropriate because of contraindications or intolerance.
Pioglitazone is also indicated for combination with insulin in type 2 diabetes mellitus adult patients with insufficient glycaemic control on insulin for whom metformin is inappropriate because of contraindications or intolerance.
After initiation of therapy with pioglitazone, patients should be reviewed after 3 to 6 months to assess adequacy of response to treatment (e. g. reduction in HbA1c). In patients who fail to show an adequate response, pioglitazone should be discontinued. In light of potential risks with prolonged therapy, prescribers should confirm at subsequent routine reviews that the benefit of pioglitazone is maintained.
Pioglitazone is indicated in the treatment of type-2 diabetes mellitus:
* as monotherapy:
- in adult patients (particularly overweight patients) inadequately controlled by diet and exercise for whom metformin is inappropriate because of contraindications or intolerance.
After initiation of therapy with pioglitazone, patients should be reviewed after 3 to 6 months to assess adequacy of response to treatment (e. g. reduction in HbA1c). In patients who fail to show an adequate response, pioglitazone should be discontinued. In light of potential risks with prolonged therapy, prescribers should confirm at subsequent routine reviews that the benefit of pioglitazone is maintained.
Pioglitazone is indicated as second or third line treatment of type 2 diabetes mellitus as described below:
* as monotherapy:
- in adult patients (particularly overweight patients) inadequately controlled by diet and exercise for whom metformin is inappropriate because of contraindications or intolerance.
* as dual oral therapy in combination with:
- metformin, in adult patients (particularly overweight patients) with insufficient glycaemic control despite maximal tolerated dose of monotherapy with metformin.
- a sulphonylurea, only in adult patients who show intolerance to metformin or for whom metformin is contraindicated, with insufficient glycaemic control despite maximal tolerated dose of monotherapy with a sulphonylurea.
* as triple oral therapy in combination with:
- metformin and a sulphonylurea, in adult patients (particularly overweight patients) with insufficient glycaemic control despite dual oral therapy.
Pioglitazone is also indicated for combination with insulin in type 2 diabetes mellitus adult patients with insufficient glycaemic control on insulin for whom metformin is inappropriate because of contraindications or intolerance (see section 4. 4).
After initiation of therapy with pioglitazone, patients should be reviewed after 3 to 6 months to assess adequacy of response to treatment (e. g. reduction in HbA1c). In patients who fail to show an adequate response, pioglitazone should be discontinued. In light of potential risks with prolonged therapy, prescribers should confirm at subsequent routine reviews that the benefit of pioglitazone is maintained (see section 4. 4).
Pioglitazone is indicated in the treatment of type 2 diabetes mellitus:
- as monotherapy
- in adult patients (particularly overweight patients) inadequately controlled by diet and exercise for whom metformin is inappropriate because of contraindications or intolerance
- as dual oral therapy in combination with
- metformin, in adult patients (particularly overweight patients) with insufficient glycaemic control despite maximal tolerated dose of monotherapy with metformin
- a sulphonylurea, only in adult patients who show intolerance to metformin or for whom metformin is contraindicated, with insufficient glycaemic control despite maximal tolerated dose of monotherapy with a sulphonylurea
- as triple oral therapy in combination with
- metformin and a sulphonylurea, in adult patients (particularly overweight patients) with insufficient glycaemic control despite dual oral therapy.
Pioglitazone is also indicated for combination with insulin in type 2 diabetes mellitus adult patients with insufficient glycaemic control on insulin for whom metformin is inappropriate because of contraindications or intolerance.
After initiation of therapy with pioglitazone, patients should be reviewed after 3 to 6 months to assess adequacy of response to treatment (e. g. reduction in HbA1c). In patients who fail to show an adequate response, pioglitazone should be discontinued. In light of potential risks with prolonged therapy, prescribers should confirm at subsequent routine reviews that the benefit of pioglitazone is maintained.
Pioglitazone is indicated as second or third line treatment of type-2 diabetes mellitus as described below:
- as monotherapy:
- in adult patients (particularly overweight patients) inadequately controlled by diet and exercise for whom metformin is inappropriate because of contraindications or intolerance;
- as dual oral therapy in combination with:
- metformin, in adult patients (particularly overweight patients) with insufficient glycaemic control despite maximal tolerated dose of monotherapy with metformin;
- a sulphonylurea, only in adult patients who show intolerance to metformin or for whom metformin is contraindicated, with insufficient glycaemic control despite maximal tolerated dose of monotherapy with a sulphonylurea;
- as triple oral therapy in combination with:
- metformin and a sulphonylurea, in adult patients (particularly overweight patients) with insufficient glycaemic control despite dual oral therapy.
Pioglitazone is also indicated for combination with insulin in type-2 diabetes mellitus adult patients with insufficient glycaemic control on insulin for whom metformin is inappropriate because of contraindications or intolerance.
After initiation of therapy with pioglitazone, patients should be reviewed after 3 to 6 months to assess adequacy of response to treatment (e. g. reduction in HbA1c). In patients who fail to show an adequate response, pioglitazone should be discontinued. In light of potential risks with prolonged therapy, prescribers should confirm at subsequent routine reviews that the benefit of pioglitazone is maintained.
Pioglitazone is indicated in the treatment of type-2 diabetes mellitus:
- as
* monotherapy: :
- in patients (particularly overweight patients) inadequately controlled by diet and exercise for whom metformin is inappropriate because of contraindications or intolerance;
- as
* dual oral therapy: in combination with:
- metformin, in patients (particularly overweight patients) with insufficient glycaemic control despite maximal tolerated dose of monotherapy with metformin;
- a sulphonylurea, only in patients who show intolerance to metformin or for whom metformin is contraindicated, with insufficient glycaemic control despite maximal tolerated dose of monotherapy with a sulphonylurea;
- as
* triple oral therapy: in combination with:
- metformin and a sulphonylurea, in patients (particularly overweight patients) with insufficient glycaemic control despite dual oral therapy.
Pioglitazone is also indicated for combination with insulin in type-2 diabetes mellitus patients with insufficient glycaemic control on insulin for whom metformin is inappropriate because of contraindications or intolerance.
Pioglitazone is indicated as second or third line treatment of type 2 diabetes mellitus as described below:
* as monotherapy:
- in adult patients (particularly overweight patients) inadequately controlled by diet and exercise for whom metformin is inappropriate because of contraindications or intolerance;
as
* dual oral therapy: in combination with
- metformin, in adult patients (particularly overweight patients) with insufficient glycaemic control despite maximal tolerated dose of monotherapy with metformin;
- a sulphonylurea, only in adult patients who show intolerance to metformin or for whom metformin is contraindicated, with insufficient glycaemic control despite maximal tolerated dose of monotherapy with a sulphonylurea;
as
* triple oral therapy: in combination with
- metformin and a sulphonylurea, in adult patients (particularly overweight patients) with insufficient glycaemic control despite dual oral therapy.
Pioglitazone is also indicated for combination with insulin in type 2 diabetes mellitus in adult patients with insufficient glycaemic control on insulin for whom metformin is inappropriate because of contraindications or intolerance.
After initiation of therapy with pioglitazone, patients should be reviewed after 3 to 6 months to assess adequacy of response to treatment (e. g. reduction in HbA1c). In patients who fail to show an adequate response, pioglitazone should be discontinued. In light of potential risks with prolonged therapy, prescribers should confirm at subsequent routine reviews that the benefit of pioglitazone is maintained.
Pioglitazone is indicated as second or third line treatment of type 2 diabetes mellitus as described below:
as monotherapy
- in adult patients (particularly overweight patients) inadequately controlled by diet and exercise for whom metformin is inappropriate because of contraindications or intolerance;
as dual oral therapy in combination with
- a sulphonylurea, only in adult patients who show intolerance to metformin or for whom metformin is contraindicated, with insufficient glycaemic control despite maximal tolerated dose of monotherapy with a sulphonylurea;
Pioglitazone is also indicated for combination with insulin in type 2 diabetes mellitus in adult patients with insufficient glycaemic control on insulin for whom metformin is inappropriate because of contraindications or intolerance.
After initiation of therapy with pioglitazone, patients should be reviewed after 3 to 6 months to assess adequacy of response to treatment (e. g. reduction in HbA1c). In patients who fail to show an adequate response, pioglitazone should be discontinued. In light of potential risks with prolonged therapy, prescribers should confirm at subsequent routine reviews that the benefit of pioglitazone is maintained.
Pioglitazone is indicated as second- or third-line treatment of type-2 diabetes mellitus as described below:
* as monotherapy: :
- in adult patients (particularly overweight patients) inadequately controlled by diet and exercise for whom metformin is inappropriate because of contraindications or intolerance;
* as dual oral therapy in combination with: :
- metformin, in adult patients (particularly overweight patients) with insufficient glycaemic control despite maximal tolerated dose of monotherapy with metformin;
- a sulphonylurea, only in adult patients who show intolerance to metformin or for whom metformin is contraindicated, with insufficient glycaemic control despite maximal tolerated dose of monotherapy with a sulphonylurea;
* as triple oral therapy in combination with: :
- metformin and a sulphonylurea, in adult patients (particularly overweight patients) with insufficient glycaemic control despite dual oral therapy.
Pioglitazone is also indicated for combination with insulin in type-2-diabetes-mellitus adult patients with insufficient glycaemic control on insulin for whom metformin is inappropriate because of contraindications or intolerance (see section 4. 4).
After initiation of therapy with pioglitazone, patients should be reviewed after three to six months to assess adequacy of response to treatment (e. g. reduction in HbA1c). In patients who fail to show an adequate response, pioglitazone should be discontinued. In light of potential risks with prolonged therapy, prescribers should confirm at subsequent routine reviews that the benefit of pioglitazone is maintained (see section 4. 4).
Pioglitazone enhances cellular responsiveness to insulin, increases insulin-dependent glucose disposal, and improves impaired glucose homeostasis. In patients with type 2 diabetes mellitus, these effects result in lower plasma glucose concentrations, lower plasma insulin concentrations, and lower HbA1c values. Significant fluid retention leading to the development/exacerbation of congestive heart failure has been reported with pioglitazone - avoid its use in patients in heart failure or at risk of developing heart failure. There is some evidence that pioglitazone may be associated with an increased risk of developing bladder cancer. Pioglitazone should not be used in patients with active bladder cancer and should be used with caution in patients with a history of bladder cancer.
Hypoglycemic Agents
Substances which lower blood glucose levels. (See all compounds classified as Hypoglycemic Agents.)
A10BG03
A10BG03
A10BG03
A10BG03
A10BG03
A10BG03
A10BG03
A10BG03
A10BG03
A10BG03
A10BG03
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
A - Alimentary tract and metabolism
A10 - Drugs used in diabetes
A10B - Blood glucose lowering drugs, excl. insulins
A10BG - Thiazolidinediones
A10BG03 - Pioglitazone
Absorption
Following oral administration of pioglitazone, peak serum concentrations are observed within 2 hours (Tmax) - food slightly delays the time to peak serum concentration, increasing Tmax to approximately 3-4 hours, but does not alter the extent of absorption. Steady-state concentrations of both parent drug and its primary active metabolites are achieved after 7 days of once-daily administration of pioglitazone. Cmax and AUC increase proportionately to administered doses.
Route of Elimination
Approximately 15-30% of orally administered pioglitazone is recovered in the urine. The bulk of its elimination, then, is presumed to be through the excretion of unchanged drug in the bile or as metabolites in the feces.
Volume of Distribution
The average apparent volume of distribution of pioglitazone is 0.63 0.41 L/kg.
Clearance
The apparent clearance of orally administered pioglitazone is 5-7 L/h.
There was no significant difference in the pharmacokinetic profile of pioglitazone in subjects with normal or with moderately impaired renal function. In patients with moderate and severe renal impairment, although mean serum concentrations of pioglitazone and its metabolites were increased, no dose adjustment is needed. After repeated oral doses of pioglitazone, mean AUC values were decreased in patients with severe renal impairment compared with healthy subjects with normal renal function for pioglitazone.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V108 362 (2016)
Following oral administration, approximately 15% to 30% of the pioglitazone dose is recovered in the urine. Renal elimination of pioglitazone is negligible, and the drug is excreted primarily as metabolites and their conjugates. It is presumed that most of the oral dose is excreted into the bile either unchanged or as metabolites and eliminated in the feces.
NIH; DailyMed. Current Medication Information for Pioglitazone Hydrochloride Tablet (Updated: December 27, 2017). Available from, as of January 26, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=8f2d7000-37ca-4e09-98ec-07d1c0354cb3
Pioglitazone is a thiazolidinedione insulin sensitizer that has shown efficacy in Type 2 diabetes and nonalcoholic fatty liver disease in humans. It may be useful for treatment of similar conditions in cats. The purpose of this study was to investigate the pharmacokinetics of pioglitazone in lean and obese cats, to provide a foundation for assessment of its effects on insulin sensitivity and lipid metabolism. Pioglitazone was administered intravenously (median 0.2 mg/kg) or orally (3 mg/kg) to 6 healthy lean (3.96 +/- 0.56 kg) and 6 obese (6.43 +/- 0.48 kg) cats, in a two by two Latin Square design with a 4-week washout period. Blood samples were collected over 24 hr, and pioglitazone concentrations were measured via a validated high-performance liquid chromatography assay. Pharmacokinetic parameters were determined using two-compartmental analysis for IV data and noncompartmental analysis for oral data. After oral administration, mean bioavailability was 55%, t(1/2) was 3.5 h, T(max) was 3.6 hr, C(max) was 2131 ng/mL, and AUC(0-8) was 15,56 ng/mL/hr. There were no statistically significant differences in pharmacokinetic parameters between lean and obese cats following either oral or intravenous administration. Systemic exposure to pioglitazone in cats after a 3 mg/kg oral dose approximates that observed in humans with therapeutic doses.
PMID:22612529 Clark MH et al; J Vet Pharmacol Ther 35 (5): 428-36 (2012)
The mean apparent volume of distribution (Vd/F) of pioglitazone following single-dose administration is 0.63 +/- 0.41 (mean +/- SD) L/kg of body weight. Pioglitazone is extensively protein bound (> 99%) in human serum, principally to serum albumin. Pioglitazone also binds to other serum proteins, but with lower affinity. M-III (keto derivative of pioglitazone) and M-IV (hydroxyl derivative of pioglitazone) are also extensively bound (> 98%) to serum albumin.
NIH; DailyMed. Current Medication Information for Pioglitazone Hydrochloride Tablet (Updated: December 27, 2017). Available from, as of January 26, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=8f2d7000-37ca-4e09-98ec-07d1c0354cb3
For more Absorption, Distribution and Excretion (Complete) data for Pioglitazone (6 total), please visit the HSDB record page.
Pioglitazone is extensively metabolized by both hydroxylation and oxidation - the resulting metabolites are also partly converted to glucuronide or sulfate conjugates. The pharmacologically active M-IV and M-III metabolites are the main metabolites found in human serum and their circulating concentrations are equal to, or greater than, those of the parent drug. The specific CYP isoenzymes involved in the metabolism of pioglitazone are CYP2C8 and, to a lesser degree, CYP3A4. There is also some evidence to suggest a contribution by extrahepatic CYP1A1.
Isoforms of cytochrome P450 (CYP) are involved in the metabolism of pioglitazone, including CYP2C8 and, to a lesser degree, CYP3A4. CYP2C9 is not significantly involved in the elimination of pioglitazone. Pioglitazone is not a strong inducer of CYP3A4, and pioglitazone was not shown to induce CYPs.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V108 362 (2016)
Pioglitazone is extensively metabolized by hydroxylation and oxidation; the metabolites also partly convert to glucuronide or sulfate conjugates. Metabolites M-III (keto derivative of pioglitazone) and M-IV (hydroxyl derivative of pioglitazone) are the major circulating active metabolites in humans.
NIH; DailyMed. Current Medication Information for Pioglitazone Hydrochloride Tablet (Updated: December 27, 2017). Available from, as of January 26, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=8f2d7000-37ca-4e09-98ec-07d1c0354cb3
Pioglitazone has known human metabolites that include 2-[6-(2-{4-[(2,4-dioxo-1,3-thiazolidin-5-yl)methyl]phenoxy}ethyl)pyridin-3-yl]acetic acid, 5-({4-[2-(5-ethylpyridin-2-yl)-2-hydroxyethoxy]phenyl}methyl)-1,3-thiazolidine-2,4-dione, and 5-[(4-{2-[5-(1-hydroxyethyl)pyridin-2-yl]ethoxy}phenyl)methyl]-1,3-thiazolidine-2,4-dione.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
The mean serum half-life of pioglitazone and its metabolites (M-III and M-IV) range from 3-7 hours and 16-24 hours, respectively.
The mean serum half-life of pioglitazone and its metabolites (M-III and M-IV) range from three to seven hours and 16 to 24 hours, respectively.
NIH; DailyMed. Current Medication Information for Pioglitazone Hydrochloride Tablet (Updated: December 27, 2017). Available from, as of January 26, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=8f2d7000-37ca-4e09-98ec-07d1c0354cb3
Pioglitazone is a selective agonist at peroxisome proliferator-activated receptor-gamma (PPAR) in target tissues for insulin action such as adipose tissue, skeletal muscle, and liver. Activation of PPAR increases the transcription of insulin-responsive genes involved in the control of glucose and lipid production, transport, and utilization. Through this mechanism, pioglitazone both enhances tissue sensitivity to insulin and reduces the hepatic production of glucose (i.e. gluconeogenesis) - insulin resistance associated with type 2 diabetes mellitus is therefore improved without an increase in insulin secretion by pancreatic beta cells.
Repeated administration of peroxisome proliferator-activated receptor gamma (PPARgamma) agonists reduces neuropathic pain-like behavior and associated changes in glial activation in the spinal cord dorsal horn. As PPARgamma is a nuclear receptor, sustained changes in gene expression are widely believed to be the mechanism of pain reduction. However, we recently reported that a single intrathecal (i.t.) injection of pioglitazone, a PPARgamma agonist, reduced hyperalgesia within 30 minutes, a time frame that is typically less than that required for genomic mechanisms. To determine the very rapid antihyperalgesic actions of PPARgamma activation, we administered pioglitazone to rats with spared nerve injury and evaluated hyperalgesia. Pioglitazone inhibited hyperalgesia within 5 minutes of injection, consistent with a nongenomic mechanism. Systemic or i.t. administration of GW9662, a PPARgamma antagonist, inhibited the antihyperalgesic actions of intraperitoneal or i.t. pioglitazone, suggesting a spinal PPAR?-dependent mechanism. To further address the contribution of nongenomic mechanisms, we blocked new protein synthesis in the spinal cord with anisomycin. When coadministered intrathecally, anisomycin did not change pioglitazone antihyperalgesia at an early 7.5-minute time point, further supporting a rapid nongenomic mechanism. At later time points, anisomycin reduced pioglitazone antihyperalgesia, suggesting delayed recruitment of genomic mechanisms. Pioglitazone reduction of spared nerve injury-induced increases in GFAP expression occurred more rapidly than expected, within 60 minutes. We are the first to show that activation of spinal PPARgamma rapidly reduces neuropathic pain independent of canonical genomic activity. We conclude that acute pioglitazone inhibits neuropathic pain in part by reducing astrocyte activation and through both genomic and nongenomic PPARgamma mechanisms.
PMID:25599238 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4329091 Griggs RB et al; Pain 156 (3): 469-82 (2015)
Pioglitazone hydrochloride is a thiazolidinedione that depends on the presence of insulin for its mechanism of action. Pioglitazone hydrochloride decreases insulin resistance in the periphery and in the liver resulting in increased insulin-dependent glucose disposal and decreased hepatic glucose output. Pioglitazone is not an insulin secretagogue. Pioglitazone is an agonist for peroxisome proliferator-activated receptor-gamma (PPARgamma). PPAR receptors are found in tissues important for insulin action such as adipose tissue, skeletal muscle, and liver. Activation of PPARgamma nuclear receptors modulates the transcription of a number of insulin responsive genes involved in the control of glucose and lipid metabolism.
NIH; DailyMed. Current Medication Information for Pioglitazone Hydrochloride Tablet (Updated: December 27, 2017). Available from, as of January 26, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=8f2d7000-37ca-4e09-98ec-07d1c0354cb3
... Thiazolidinediones reduce insulin resistance not only in type 2 diabetes but also in non-diabetic conditions associated with insulin resistance such as obesity. The mechanism of action involves binding to the peroxisome proliferator-activated receptor (PPAR)gamma, a transcription factor that regulates the expression of specific genes especially in fat cells but also in other tissues. It is likely that thiazolidinediones primarily act in adipose tissue where PPARgamma is predominantly expressed. Thiazolidinediones have been shown to interfere with expression and release of mediators of insulin resistance originating in adipose tissue (e.g. free fatty acids, adipocytokines such as tumor necrosis factor alpha, resistin, adiponectin) in a way that results in net improvement of insulin sensitivity (i.e. in muscle and liver). Nevertheless, a direct molecular effect in skeletal muscle cannot be excluded. ...
PMID:12173692 Stumvoll M, Haring HU; Ann Med 34 (3): 217-24 (2002)
Pioglitazone, a full peroxisome proliferator-activated receptor (PPAR)-gamma agonist, improves insulin sensitivity by increasing circulating adiponectin levels. However, the molecular mechanisms by which pioglitazone induces insulin sensitization are not fully understood. In this study, we investigated whether pioglitazone improves insulin resistance via upregulation of either 2 distinct receptors for adiponectin (AdipoR1 or AdipoR2) expression in 3T3-L1 adipocytes. Glucose uptake was evaluated by 2-[(3)H] deoxy-glucose uptake assay in 3T3-L1 adipocytes with pioglitazone treatment. AdipoR1 and AdipoR2 mRNA expressions were analyzed by qRT-PCR. /The investigators/ first confirmed that pioglitazone significantly increased insulin-induced 2-deoxyglucose (2-DOG) uptake in 3T3-L1 adipocytes. Next, we investigated the mRNA expression and regulation of AdipoR1 and AdipoR2 after treatment with pioglitazone. Interestingly, pioglitazone significantly induced AdipoR2 expression but it did not affect AdipoR1 expression. In addition, adenovirus-mediated PPARgamma expression significantly enhanced the effects of pioglitazone on insulin-stimulated 2-DOG uptake and AdipoR2 expression in 3T3-L1 adipocytes. These data suggest that pioglitazone enhances adiponectin's autocrine and paracrine actions in 3T3-L1 adipocytes via upregulation of PPARgamma-mediated AdipoR2 expression. Furthermore, we found that pioglitazone significantly increased AMP-activated protein kinase (AMPK) phosphorylation in insulin-stimulated 3T3-L1 adipocytes, but it did not lead to the phosphorylation of IRS-1, Akt, or protein kinase ... Pioglitazone increases insulin sensitivity, at least partly, by PPARgamma-AdipoR2-mediated AMPK phosphorylation in 3T3-L1 adipocytes. In conclusion, the upregulation of AdipoR2 expression may be one of the mechanisms by which pioglitazone improves insulin resistance in 3T3-L1 adipocytes.
PMID:21514306 Kudoh A et al; Life Sci 88 (23-24): 1055-62 (2011)
For more Mechanism of Action (Complete) data for Pioglitazone (6 total), please visit the HSDB record page.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
34
PharmaCompass offers a list of Pioglitazone API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Pioglitazone manufacturer or Pioglitazone supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Pioglitazone manufacturer or Pioglitazone supplier.
PharmaCompass also assists you with knowing the Pioglitazone API Price utilized in the formulation of products. Pioglitazone API Price is not always fixed or binding as the Pioglitazone Price is obtained through a variety of data sources. The Pioglitazone Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Pioglitazone manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Pioglitazone, including repackagers and relabelers. The FDA regulates Pioglitazone manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Pioglitazone API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Pioglitazone manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Pioglitazone supplier is an individual or a company that provides Pioglitazone active pharmaceutical ingredient (API) or Pioglitazone finished formulations upon request. The Pioglitazone suppliers may include Pioglitazone API manufacturers, exporters, distributors and traders.
click here to find a list of Pioglitazone suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Pioglitazone Drug Master File in Japan (Pioglitazone JDMF) empowers Pioglitazone API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Pioglitazone JDMF during the approval evaluation for pharmaceutical products. At the time of Pioglitazone JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Pioglitazone suppliers with JDMF on PharmaCompass.
A Pioglitazone written confirmation (Pioglitazone WC) is an official document issued by a regulatory agency to a Pioglitazone manufacturer, verifying that the manufacturing facility of a Pioglitazone active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Pioglitazone APIs or Pioglitazone finished pharmaceutical products to another nation, regulatory agencies frequently require a Pioglitazone WC (written confirmation) as part of the regulatory process.
click here to find a list of Pioglitazone suppliers with Written Confirmation (WC) on PharmaCompass.
Pioglitazone Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Pioglitazone GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Pioglitazone GMP manufacturer or Pioglitazone GMP API supplier for your needs.
A Pioglitazone CoA (Certificate of Analysis) is a formal document that attests to Pioglitazone's compliance with Pioglitazone specifications and serves as a tool for batch-level quality control.
Pioglitazone CoA mostly includes findings from lab analyses of a specific batch. For each Pioglitazone CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Pioglitazone may be tested according to a variety of international standards, such as European Pharmacopoeia (Pioglitazone EP), Pioglitazone JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Pioglitazone USP).

