



API Suppliers

US DMFs Filed

CEP/COS Certifications

JDMFs Filed
0
EU WC
0
Listed Suppliers
0
0

USA (Orange Book)
0

Europe
0

Canada

Australia
0

South Africa
Uploaded Dossiers
U.S. Medicaid
0
Annual Reports
0
0
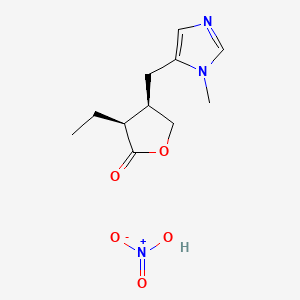

1. Hydrochloride, Pilocarpine
2. Isopilocarpine
3. Isoptocarpine
4. Nitrate, Pilocarpine
5. Ocusert
6. Pilocarpine
7. Pilocarpine Hydrochloride
8. Pilocarpine Mononitrate, (3s-cis)-isomer
9. Pilocarpine, Monohydrochloride, (3s-cis)-isomer
10. Salagen
1. 148-72-1
2. Pilagan
3. Pilofrin
4. Pilocarpine Mononitrate
5. Pilocarpine Nitrate Salt
6. Pilocarpine (nitrate)
7. Pilocarpinum Nitricum
8. Pilocarpine Subnitrate
9. Pilocarpine Nitrate [usp]
10. Nsc-757282
11. 2(3h)-furanone, 3-ethyldihydro-4-[(1-methyl-1h-imidazol-5-yl)methyl]-, (3s,4r)-, Nitrate (1:1)
12. Mls000069669
13. M20t465h6j
14. (3s,4r)-3-ethyl-4-((1-methyl-1h-imidazol-5-yl)methyl)dihydrofuran-2(3h)-one Nitrate
15. Smr000058498
16. Pilocarpine Nitrate (usp)
17. (3s,4r)-3-ethyl-4-[(3-methylimidazol-4-yl)methyl]oxolan-2-one;nitric Acid
18. Pilocarpini Nitras
19. Pilocarpin Nitrate
20. 2(3h)-furanone, 3-ethyldihydro-4-((1-methyl-1h-imidazol-5-yl)methyl)-, (3s-cis)-, Mononitrate
21. Sr-01000075339
22. P. V. Carpine Liquifilm
23. Unii-m20t465h6j
24. Sr-01000000054
25. 2(3h)-furanone, 3-ethyldihydro-4-((1-methyl-1h-imidazol-5-yl)methyl)-, (3s,4r)-, Nitrate (1:1)
26. Prestwick_282
27. Einecs 205-723-7
28. Pilagan (tn)
29. Mfcd00078497
30. Pilocarpine, Mononitrate
31. Regid855878
32. Opera_id_1243
33. Schembl41889
34. Spectrum1500487
35. Chembl1213136
36. Pilocarpine Nitrate [mi]
37. Hms501b20
38. Dtxsid80883277
39. Hms1569i20
40. Hms1920h14
41. Hms2091p18
42. Hms2096i20
43. Hms2235p11
44. Hms3262p22
45. Hms3713i20
46. Pharmakon1600-01500487
47. Pilocarpine Nitrate [vandf]
48. Hy-b1006
49. Pilocarpine Nitrate [mart.]
50. Pilocarpinum Nitricum [hpus]
51. Tox21_500960
52. Ccg-38830
53. Nsc757282
54. Pilocarpine Nitrate [usp-rs]
55. Pilocarpine Nitrate [who-dd]
56. Pilocarpine Nitrate [who-ip]
57. Akos016009842
58. Cs-4503
59. Lp00960
60. Nsc 757282
61. Ncgc00094261-01
62. Ncgc00261645-01
63. Pilocarpine Nitrate [ep Impurity]
64. Pilocarpine Nitrate [ep Monograph]
65. Pilocarpini Nitras [who-ip Latin]
66. Pilocarpine Nitrate [usp Monograph]
67. Eu-0100960
68. D05478
69. P 6628
70. T71804
71. Pilocarpine Nitrate Salt, >=98% (tlc), Powder
72. Sr-01000000054-7
73. Sr-01000075339-2
74. Sr-01000075339-5
75. Pilocarpine Nitrate Salt, Tested According To Ph.eur.
76. Pilocarpine Nitrate, Meets Usp Testing Specifications
77. Q27283365
78. Sr-01000075339-10
79. Pilocarpine Nitrate, British Pharmacopoeia (bp) Reference Standard
80. Pilocarpine Nitrate, European Pharmacopoeia (ep) Reference Standard
81. Pilocarpine Nitrate, United States Pharmacopeia (usp) Reference Standard
82. Pilocarpine Nitrate For System Suitability, European Pharmacopoeia (ep) Reference Standard
83. Pilocarpine Nitrate, Pharmaceutical Secondary Standard; Certified Reference Material
84. 2(3h)-furanone, 3-ethyldihydro-4-((1-methyl-1h-imidazol-5-yl)methyl)-, (3s,4r)-, Mononitrate
| Molecular Weight | 271.27 g/mol |
|---|---|
| Molecular Formula | C11H17N3O5 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 3 |
| Exact Mass | 271.11682065 g/mol |
| Monoisotopic Mass | 271.11682065 g/mol |
| Topological Polar Surface Area | 110 Ų |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 270 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Miotics
Agents causing contraction of the pupil of the eye. Some sources use the term miotics only for the parasympathomimetics but any drug used to induce miosis is included here. (See all compounds classified as Miotics.)
Muscarinic Agonists
Drugs that bind to and activate muscarinic cholinergic receptors (RECEPTORS, MUSCARINIC). Muscarinic agonists are most commonly used when it is desirable to increase smooth muscle tone, especially in the GI tract, urinary bladder and the eye. They may also be used to reduce heart rate. (See all compounds classified as Muscarinic Agonists.)


