


API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
0
Listed Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
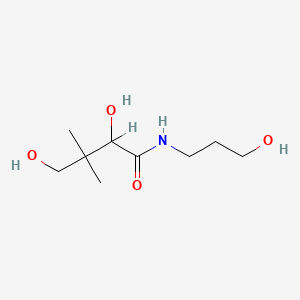

1. (+)-2,4-dihydroxy-n-(3-hydroxypropyl)-3,3-dimethylbutyramide
2. 2,4-dihydroxy-n-(3-hydroxypropyl)-3,3-dimethylbutanamide
3. Bepanthen
4. Butanamide, 2,4-dihydroxy-n-(3-hydroxypropyl)-3,3-dimethyl-, (+--)-
5. Corneregel
6. D-panthenol
7. Dexpanthenol
8. Dexpanthenol Heumann
9. Dl-panthenol
10. Ilopan
11. Marolderm
12. Nasenspray Ratiopharm Panthenol
13. Nasicur
14. Otriven Dexpanthenol
15. Pan Rhinol
16. Pan-ophtal
17. Panthenol Braun
18. Panthenol Jenapharm
19. Panthenol Law
20. Panthenol Lichtenstein
21. Panthenol Von Ct
22. Panthenol-ratiopharm
23. Panthoderm
24. Panthogenat
25. Pantothenol
26. Repa-ophtal
27. Rhinoclir
28. Siozwo Sana
29. Ucee D
30. Urupan
31. Wund- Und Heilsalbe Law
1. Dl-panthenol
2. 16485-10-2
3. Dl-pantothenol
4. Dl-pantothenyl Alcohol
5. 2,4-dihydroxy-n-(3-hydroxypropyl)-3,3-dimethylbutanamide
6. Butanamide, 2,4-dihydroxy-n-(3-hydroxypropyl)-3,3-dimethyl-
7. Panthenol, Racemic
8. Panthenolum
9. 2,4-dihydroxy-n-(3-hydroxypropyl)-3,3-dimethylbutyramide
10. 62507-76-0
11. Nsc-759899
12. Wv9cm0o67z
13. Pantenol
14. Pantenolo
15. (+-)-pantothenyl Alcohol
16. Dl-pantothenol;dl-pantothenyl Alcohol
17. Alcool Dl-pantotenilico
18. Panthenol , Dl-form
19. Butanamide, 2,4-dihydroxy-n-(3-hydroxypropyl)-3,3-dimethyl-, (+)- (9ci)
20. Pantenolo [dcit]
21. Pantenol [inn-spanish]
22. Panthenolum [inn-latin]
23. Component Of Zentinic
24. Dexpanthenol [usan)
25. Smr000857333
26. Alcool Dl-pantotenilico [italian]
27. Sr-05000001760
28. Einecs 240-540-6
29. Mfcd00002944
30. Unii-wv9cm0o67z
31. Nsc302962
32. D,l- Panthenol
33. Nsc-302962
34. Ncgc00186658-01
35. Pantothenylol Alcohol
36. Panthenol [usan:usp:inn:ban:jan]
37. 2,3-dimethylbutyramide
38. Dl-panthenol, 99%
39. Panthenol (usp/inn)
40. Panthenol [inn]
41. Panthenol, Dl-
42. Panthenol [inci]
43. Panthenol [usan]
44. (+-)-2,4-dihydroxy-n-(3-hydroxypropyl)-3,3-dimethylbutyramide
45. Panthenol [vandf]
46. Panthenol [mart.]
47. Ec 240-540-6
48. Dl-panthenol [fcc]
49. Panthenol [who-dd]
50. Dsstox_cid_24598
51. Dsstox_rid_80341
52. Dsstox_gsid_44598
53. Schembl15567
54. Dl-panthenol [vandf]
55. (r)-2,4-dihydroxy-3,3-dimethylbutyric 3-hydroxypropylamide
56. Mls001336015
57. Mls001336016
58. Chembl1371937
59. D-(+)-2,3-dimethylbutyramide
60. Dtxsid3044598
61. Panthenol [usp Monograph]
62. Dexpanthenol (pantothenyl Alcohol)
63. Hms2093b14
64. Hms2234m16
65. Hms3371m11
66. Pharmakon1600-01505420
67. Pharmakon1600-01505656
68. (+/-)-pantothenyl Alcohol
69. Hy-b1024
70. Panthenol, Racemic [usp-rs]
71. Tox21_302660
72. Butanamide, 2,4-dihydroxy-n-(3-hydroxypropyl)-3,3-dimethyl-, (+-)-
73. Butyramide, 2,4-dihydroxy-n-(3-hydroxypropyl)-3,3-dimethyl-, Dl-
74. Nsc759127
75. Nsc759899
76. S4566
77. Stl453540
78. Akos015841508
79. Butanamide, 2,4-dihydroxy-n-(3-hydroxypropyl)-3,3-dimethyl-, (+/-)-
80. Ccg-213502
81. Cs-4541
82. Nsc 759899
83. Nsc-759127
84. Sb44496
85. Ncgc00256864-01
86. As-56363
87. Da-09547
88. Sbi-0206818.p001
89. Cas-16485-10-2
90. Db-056494
91. Ft-0625499
92. Ft-0625596
93. Ft-0693817
94. P1318
95. A19436
96. D03726
97. H11256
98. Ab00918367_05
99. A810597
100. Butyramide, 2,4-dihydroxy-n-(3-hydroxypropyl)-3
101. Q196473
102. Q-201031
103. Sr-05000001760-1
104. Sr-05000001760-3
105. 3-(2,4-dihydroxy-3,3-dimethylbutyramido)-1-propanol
106. Brd-a59413292-001-04-1
107. Butanamide,4-dihydroxy-n-(3-hydroxypropyl)-3,3-dimethyl-
108. (+/-)-2,4-dihydroxy-3,3-dimethylbutyric 3-hydroxypropylamide
109. 3,3-dimethyl-2,4-bis(oxidanyl)-n-(3-oxidanylpropyl)butanamide
110. D-2,4-dihydroxy-3,3-dimethyl-n-(3-hydroxypropyl)butyramide
111. Dl-2 4-dihydroxy-n-(3-hydroxypropyl)-3 3-dimethylbutyramide
112. (+/-)-2,4-dihydroxy-n-(3-hydroxypropyl)-3,3-dimethylbutyramide
113. (r)-(+)-2,4-dihydroxy-n-(3-hydroxypropyl)-3,3-dimethylbutyramide
114. Butanamide,4-dihydroxy-n-(3-hydroxypropyl)-3,3-dimethyl-, (r)-
115. Butyramide,4-dihydroxy-n-(3-hydroxypropyl)-3,3-dimethyl-, D-(+)-
116. Panthenol, Racemic, United States Pharmacopeia (usp) Reference Standard
117. (+/-)-alpha,gamma-dihydroxy-n-(3-hydroxypropyl)-beta,beta-dimethylbutyramide
118. Butyramide, .alpha.,.gamma.-dihydroxy-n-(3-hydroxypropyl)-.beta.,.beta.-dimethyl-
119. Butyramide, 2,4-dihydroxy-n-(3-hydroxypropyl)-3,3-dimethyl-, (+/-)-
| Molecular Weight | 205.25 g/mol |
|---|---|
| Molecular Formula | C9H19NO4 |
| XLogP3 | -0.9 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 6 |
| Exact Mass | 205.13140809 g/mol |
| Monoisotopic Mass | 205.13140809 g/mol |
| Topological Polar Surface Area | 89.8 Ų |
| Heavy Atom Count | 14 |
| Formal Charge | 0 |
| Complexity | 182 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Panthenol (containing a racemic mixture of dexpanthenol and levopanthenol) is not currently available in any FDA-approved products and therefore does not have an indication. Please see [DB09357] for FDA-approved uses of the dextrorotatory form of Panthenol.
Pantothenic acid is a precursor of coenzyme A, which serves as a cofactor for a variety of enzyme-catalyzed reactions involving transfer of acetyl groups. The final step in the synthesis of acetylcholine consists of the choline acetylase transfer of acetyl group from acetylcoenzyme A to choline. Acetylcholine is the neurohumoral transmitter in the parasympathetic system and as such maintains the normal functions of the intestine. Decrease in acetylcholine content would result in decreased peristalsis and in extreme cases adynamic ileus.
D03AX03
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
Panthenol is an alcohol derivative of pantothenic acid, a component of the B complex vitamins and an essential component of a normally functioning epithelium. Dexpanthenol, the active form of panthenol, is enzymatically cleaved to form pantothenic acid (Vitamin B5), which is an essential component of Coenzyme A that acts as a cofactor in many enzymatic reactions that are important for protein metabolism in the epithelium. Dermatological effects of the topical use of dexpanthenol include increased fibroblast proliferation and accelerated re-epithelialization in wound healing. Furthermore, it acts as a topical protectant, moisturizer, and has demonstrated anti-inflammatory properties.


