


API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0

USA (Orange Book)

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
Annual Reports
0
0
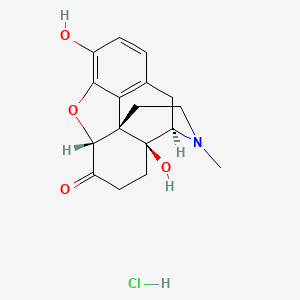

1. Numorphan
2. Opana
3. Oxymorphone
4. Oxymorphone Hcl
1. Oxymorphone Hcl
2. 357-07-3
3. Opana Er
4. Oxymorphone Hydrochloride [usp]
5. 5y2ei94nbc
6. Oxymorphone Hcl Narcotic Analgesic
7. Oxymorphone Hydrochloride (usp)
8. Oxymorphinone Hydrochloride
9. (4r,4as,7ar,12bs)-4a,9-dihydroxy-3-methyl-2,4,5,6,7a,13-hexahydro-1h-4,12-methanobenzofuro[3,2-e]isoquinolin-7-one;hydrochloride
10. Einecs 206-610-5
11. Unii-5y2ei94nbc
12. Numorphan (tn)
13. Opana (tn)
14. 4,5-alpha-epoxy-3,14-dihydroxy-17-methylmorphinan-6-one Hydrochloride
15. 4,5alpha-epoxy-3,14-dihydroxy-17-methylmorphinan-6-one Hydrochloride
16. Schembl41770
17. Chebi:7866
18. Chembl1200794
19. Dtxsid10189214
20. Oxymorphone Hydrochloride [mi]
21. (5alpha)-4,5-epoxy-3,14-dihydroxy-17-methylmorphinan-6-one Hydrochloride
22. Morphinan-6-one, 3,14-dihydroxy-4,5-alpha-epoxy-17-methyl-, Hydrochloride
23. Morphinan-6-one, 4,5-epoxy-3,14-dihydroxy-17-methyl-, Hydrochloride, (5alpha)-
24. Oxymorphone Hydrochloride [mart.]
25. Oxymorphone Hydrochloride [vandf]
26. Oxymorphone Hydrochloride [who-dd]
27. Oxymorphone Hydrochloride [green Book]
28. D00844
29. Oxymorphone Hydrochloride [orange Book]
30. Oxymorphone Hydrochloride [usp Monograph]
31. Q27263031
32. 4,5.alpha.-epoxy-3,14-dihydroxy-17-methylmorphinan-6-one Hydrochloride
33. Morphinan-6-one, 4,5-epoxy-3,14-dihydroxy-17-methyl-, Hydrochloride, (5.alpha.)-
| Molecular Weight | 337.8 g/mol |
|---|---|
| Molecular Formula | C17H20ClNO4 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 0 |
| Exact Mass | 337.1080858 g/mol |
| Monoisotopic Mass | 337.1080858 g/mol |
| Topological Polar Surface Area | 70 Ų |
| Heavy Atom Count | 23 |
| Formal Charge | 0 |
| Complexity | 539 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 6 | |
|---|---|
| Drug Name | Opana |
| PubMed Health | Oxymorphone (Injection) |
| Drug Classes | Analgesic, Anesthetic Adjunct |
| Drug Label | OPANA ER (oxymorphone hydrochloride) extended-release, is a semi-synthetic opioid analgesic supplied in 5 mg, 10 mg, 20 mg, and 40 mg tablet strengths for oral administration. The tablet strength describes the amount of oxymorphone hydrochloride per... |
| Active Ingredient | Oxymorphone hydrochloride |
| Dosage Form | Tablet; Injectable |
| Route | Injection; Oral |
| Strength | 1mg/ml; 5mg; 10mg |
| Market Status | Prescription |
| Company | Endo Pharms |
| 2 of 6 | |
|---|---|
| Drug Name | Opana er |
| PubMed Health | Oxymorphone (By mouth) |
| Drug Classes | Analgesic |
| Drug Label | OPANA ER (oxymorphone hydrochloride) extended-release is a semi-synthetic opioid analgesic supplied in 5 mg, 7.5 mg, 10 mg, 15 mg, 20 mg, 30 mg, and 40 mg tablet strengths for oral administration. The tablet strength describes the amount of oxymorpho... |
| Active Ingredient | Oxymorphone hydrochloride |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 7.5mg; 30mg; 15mg; 5mg; 10mg; 40mg; 20mg |
| Market Status | Prescription |
| Company | Endo Pharms |
| 3 of 6 | |
|---|---|
| Drug Name | Oxymorphone hydrochloride |
| Drug Label | OPANA ER (oxymorphone hydrochloride) extended-release, is a semi-synthetic opioid analgesic supplied in 5 mg, 10 mg, 20 mg, and 40 mg tablet strengths for oral administration. The tablet strength describes the amount of oxymorphone hydrochloride per... |
| Active Ingredient | Oxymorphone hydrochloride |
| Dosage Form | Tablet, extended release; Tablet |
| Route | Oral |
| Strength | 7.5mg; 30mg; 15mg; 5mg; 10mg; 40mg; 20mg |
| Market Status | Prescription |
| Company | Avanthi; Teva; Mallinckrodt; Roxane; Actavis Elizabeth; Impax Labs |
| 4 of 6 | |
|---|---|
| Drug Name | Opana |
| PubMed Health | Oxymorphone (Injection) |
| Drug Classes | Analgesic, Anesthetic Adjunct |
| Drug Label | OPANA ER (oxymorphone hydrochloride) extended-release, is a semi-synthetic opioid analgesic supplied in 5 mg, 10 mg, 20 mg, and 40 mg tablet strengths for oral administration. The tablet strength describes the amount of oxymorphone hydrochloride per... |
| Active Ingredient | Oxymorphone hydrochloride |
| Dosage Form | Tablet; Injectable |
| Route | Injection; Oral |
| Strength | 1mg/ml; 5mg; 10mg |
| Market Status | Prescription |
| Company | Endo Pharms |
| 5 of 6 | |
|---|---|
| Drug Name | Opana er |
| PubMed Health | Oxymorphone (By mouth) |
| Drug Classes | Analgesic |
| Drug Label | OPANA ER (oxymorphone hydrochloride) extended-release is a semi-synthetic opioid analgesic supplied in 5 mg, 7.5 mg, 10 mg, 15 mg, 20 mg, 30 mg, and 40 mg tablet strengths for oral administration. The tablet strength describes the amount of oxymorpho... |
| Active Ingredient | Oxymorphone hydrochloride |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 7.5mg; 30mg; 15mg; 5mg; 10mg; 40mg; 20mg |
| Market Status | Prescription |
| Company | Endo Pharms |
| 6 of 6 | |
|---|---|
| Drug Name | Oxymorphone hydrochloride |
| Drug Label | OPANA ER (oxymorphone hydrochloride) extended-release, is a semi-synthetic opioid analgesic supplied in 5 mg, 10 mg, 20 mg, and 40 mg tablet strengths for oral administration. The tablet strength describes the amount of oxymorphone hydrochloride per... |
| Active Ingredient | Oxymorphone hydrochloride |
| Dosage Form | Tablet, extended release; Tablet |
| Route | Oral |
| Strength | 7.5mg; 30mg; 15mg; 5mg; 10mg; 40mg; 20mg |
| Market Status | Prescription |
| Company | Avanthi; Teva; Mallinckrodt; Roxane; Actavis Elizabeth; Impax Labs |
Analgesics, Opioid
Compounds with activity like OPIATE ALKALOIDS, acting at OPIOID RECEPTORS. Properties include induction of ANALGESIA or NARCOSIS. (See all compounds classified as Analgesics, Opioid.)
Narcotics
Agents that induce NARCOSIS. Narcotics include agents that cause somnolence or induced sleep (STUPOR); natural or synthetic derivatives of OPIUM or MORPHINE or any substance that has such effects. They are potent inducers of ANALGESIA and OPIOID-RELATED DISORDERS. (See all compounds classified as Narcotics.)
Adjuvants, Anesthesia
Agents that are administered in association with anesthetics to increase effectiveness, improve delivery, or decrease required dosage. (See all compounds classified as Adjuvants, Anesthesia.)




