
Synopsis
Synopsis
0
JDMF
0
VMF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
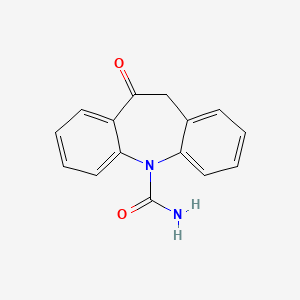

1. 10,11-dihydro-10-oxo-5h-dibenz(b,f)azepine-5-carboxamide
2. Gp 47680
3. Timox
4. Trileptal
1. 28721-07-5
2. Trileptal
3. Oxcarbamazepine
4. Oxcarbazepina
5. Oxcarbazepinum
6. Gp 47680
7. 10-oxo-10,11-dihydro-5h-dibenzo[b,f]azepine-5-carboxamide
8. 5-oxo-6h-benzo[b][1]benzazepine-11-carboxamide
9. Kin-493
10. Gp-47680
11. Oxtellar Xr
12. 10,11-dihydro-10-oxo-5h-dibenz(b,f)azepine-5-carboxamide
13. 10,11-dihydro-10-oxo-5h-dibenz[b,f]azepine-5-carboxamide
14. Spn-804
15. 5h-dibenz[b,f]azepine-5-carboxamide, 10,11-dihydro-10-oxo-
16. Mls000084586
17. 9-oxo-2-azatricyclo[9.4.0.0^{3,8}]pentadeca-1(11),3(8),4,6,12,14-hexaene-2-carboxamide
18. 5h-dibenz(b,f)azepine-5-carboxamide, 10,11-dihydro-10-oxo-
19. Nsc-758693
20. Chembl1068
21. Smr000048684
22. Chebi:7824
23. Vzi5b1w380
24. Timox
25. Ncgc00065934-02
26. Oxcarbazepine [inn]
27. Dsstox_cid_25703
28. Dsstox_rid_81075
29. Dsstox_gsid_45703
30. Oxcarbazepinum [inn-latin]
31. Oxcarbazepina [inn-spanish]
32. Oxcarbazepime
33. Epilexter
34. Epliga
35. Trileptal (tn)
36. Cas-28721-07-5
37. Sr-01000612612
38. Einecs 249-188-8
39. Unii-vzi5b1w380
40. Oxcarbazepin
41. Hsdb 7524
42. Oxtellar (tn)
43. Oxcarbazepine [usan:usp:inn:ban]
44. Mfcd00865307
45. Ocbz
46. Oxcarbazepine Solution
47. Oxcarbazepine- Bio-x
48. Spectrum_001675
49. Opera_id_818
50. Regid866068
51. Spectrum2_000483
52. Spectrum3_001669
53. Spectrum4_000634
54. Spectrum5_001869
55. O0363
56. Oxcarbazepine [mi]
57. Oxcarbazepine [jan]
58. Oxcarbazepine [hsdb]
59. Oxcarbazepine [usan]
60. Schembl35129
61. Bspbio_003457
62. Kbiogr_001248
63. Kbioss_002155
64. Oxcarbazepine [vandf]
65. Cid_34312
66. Mls000759520
67. Mls001201742
68. Mls001424025
69. Mls006011855
70. Bidd:gt0078
71. Oxcarbazepine [mart.]
72. Spectrum1504243
73. Spbio_000345
74. Oxcarbazepine [usp-rs]
75. Oxcarbazepine [who-dd]
76. Gtpl7254
77. Oxcarbazepine (jan/usp/inn)
78. Zinc4724
79. Dtxsid0045703
80. Bdbm34179
81. Kbio2_002155
82. Kbio2_004723
83. Kbio2_007291
84. Kbio3_002677
85. Oxcarbazepine, Analytical Standard
86. 10,11-dihydro-10-oxo-5h-dibenzo[b,f]azepine-5-carboxamide
87. Hms1922h17
88. Hms2051o04
89. Hms2090f13
90. Hms2093e10
91. Hms2231b12
92. Hms3369j22
93. Hms3393o04
94. Hms3657o11
95. Hms3713i10
96. Hms3884k13
97. Pharmakon1600-01504243
98. Oxcarbazepine [orange Book]
99. Bcp28260
100. Bcp33398
101. Hy-b0114
102. Oxcarbazepine [ep Monograph]
103. Tox21_110983
104. Ccg-39509
105. Nsc758693
106. Oxcarbazepine [usp Monograph]
107. S1391
108. Stk594696
109. Akos005516529
110. Tox21_110983_1
111. Ac-3483
112. Cs-1869
113. Db00776
114. Ks-5197
115. Nc00088
116. Nsc 758693
117. Oxcarbazepine, >=98% (hplc), Solid
118. Ncgc00065934-03
119. Ncgc00065934-04
120. Ncgc00065934-05
121. Ncgc00065934-06
122. Bo164187
123. Sbi-0206772.p001
124. Gp-47-680
125. Am20040094
126. Ft-0630543
127. Ft-0673414
128. Sw197468-3
129. A13943
130. C07492
131. D00533
132. M06310
133. Ab00393017-12
134. Ab00393017-14
135. Ab00393017_15
136. Ab00393017_16
137. 721o075
138. Q176301
139. Sr-01000612612-4
140. Sr-01000612612-6
141. W-107033
142. Brd-k04196797-001-12-9
143. 10-oxo-10,11-dihydro-5h-dibenz(b,f)azepin-5-carboxamide
144. 5-carbamoyl-10-oxo-10,11-dihydro-5h-dibenz[b,f]azepine
145. 10,11-dihydro-10-oxo-5h-dibenz[b,f]azepin-5-carbonsaeureamid
146. 10-oxo-10,11-dihydro-5h-dibenzo[b,f]azepine-5-carboxamide #
147. Oxcarbazepine, European Pharmacopoeia (ep) Reference Standard
148. 10,11-dihydro-10-oxo-5h-dibenzo(z)[b,f]azepine-5-carboxamide
149. Oxcarbazepine, United States Pharmacopeia (usp) Reference Standard
150. 10-oxo Carbazepine; Oxecarb; 10,11-dihdyro-10-oxo-5h-dibenz[b,f]azepine-5-carboxamide
151. Oxcarbazepine, Pharmaceutical Secondary Standard; Certified Reference Material
152. Oxcarbazepine Solution, 1.0 Mg/ml In Acetonitrile, Ampule Of 1 Ml, Certified Reference Material
153. Oxcarbazepine-13c6 Solution, 100 Mug/ml In Acetonitrile, Ampule Of 1 Ml, Certified Reference Material
| Molecular Weight | 252.27 g/mol |
|---|---|
| Molecular Formula | C15H12N2O2 |
| XLogP3 | 1.7 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 252.089877630 g/mol |
| Monoisotopic Mass | 252.089877630 g/mol |
| Topological Polar Surface Area | 63.4 Ų |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 382 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 6 | |
|---|---|
| Drug Name | Oxcarbazepine |
| PubMed Health | Oxcarbazepine (By mouth) |
| Drug Classes | Anticonvulsant |
| Drug Label | Oxcarbazepine is an antiepileptic drug available as 150 mg, 300 mg and 600 mg film-coated tablets for oral administration. Oxcarbazepine is 10,11-Dihydro-10-oxo-5H-dibenz[b,f]azepine-5-carboxamide, and its structural formula isOxcarbazepine is a whit... |
| Active Ingredient | Oxcarbazepine |
| Dosage Form | Tablet; Suspension |
| Route | Oral |
| Strength | 150mg; 600mg; 300mg; 300mg/5ml |
| Market Status | Prescription |
| Company | Amneal Pharms; Ranbaxy; Breckenridge Pharm; Apotex; Sun Pharm Inds; Taro; Roxane; Glenmark Generics; Cadista Pharms |
| 2 of 6 | |
|---|---|
| Drug Name | Oxtellar xr |
| Drug Label | Oxtellar XR is an antiepileptic drug (AED). Oxtellar XR extended-release tablets contain oxcarbazepine for once-a-day oral administration. Oxcarbazepine is 10,11-Dihydro-10-oxo-5H-dibenz[b,f]-azepine-5-carboxamide, and its structural formula is... |
| Active Ingredient | Oxcarbazepine |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 150mg; 600mg; 300mg |
| Market Status | Prescription |
| Company | Supernus Pharms |
| 3 of 6 | |
|---|---|
| Drug Name | Trileptal |
| PubMed Health | Oxcarbazepine (By mouth) |
| Drug Classes | Anticonvulsant |
| Drug Label | Trileptal is an antiepileptic drug available as 150 mg, 300 mg and 600 mg film-coated tablets for oral administration. Trileptal is also available as a 300 mg/5 mL (60mg/mL) oral suspension. Oxcarbazepine is 10,11-Dihydro-10-oxo-5H-dibenz[b,f]azepi... |
| Active Ingredient | Oxcarbazepine |
| Dosage Form | Tablet; Suspension |
| Route | Oral |
| Strength | 150mg; 600mg; 300mg; 300mg/5ml |
| Market Status | Prescription |
| Company | Novartis |
| 4 of 6 | |
|---|---|
| Drug Name | Oxcarbazepine |
| PubMed Health | Oxcarbazepine (By mouth) |
| Drug Classes | Anticonvulsant |
| Drug Label | Oxcarbazepine is an antiepileptic drug available as 150 mg, 300 mg and 600 mg film-coated tablets for oral administration. Oxcarbazepine is 10,11-Dihydro-10-oxo-5H-dibenz[b,f]azepine-5-carboxamide, and its structural formula isOxcarbazepine is a whit... |
| Active Ingredient | Oxcarbazepine |
| Dosage Form | Tablet; Suspension |
| Route | Oral |
| Strength | 150mg; 600mg; 300mg; 300mg/5ml |
| Market Status | Prescription |
| Company | Amneal Pharms; Ranbaxy; Breckenridge Pharm; Apotex; Sun Pharm Inds; Taro; Roxane; Glenmark Generics; Cadista Pharms |
| 5 of 6 | |
|---|---|
| Drug Name | Oxtellar xr |
| Drug Label | Oxtellar XR is an antiepileptic drug (AED). Oxtellar XR extended-release tablets contain oxcarbazepine for once-a-day oral administration. Oxcarbazepine is 10,11-Dihydro-10-oxo-5H-dibenz[b,f]-azepine-5-carboxamide, and its structural formula is... |
| Active Ingredient | Oxcarbazepine |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 150mg; 600mg; 300mg |
| Market Status | Prescription |
| Company | Supernus Pharms |
| 6 of 6 | |
|---|---|
| Drug Name | Trileptal |
| PubMed Health | Oxcarbazepine (By mouth) |
| Drug Classes | Anticonvulsant |
| Drug Label | Trileptal is an antiepileptic drug available as 150 mg, 300 mg and 600 mg film-coated tablets for oral administration. Trileptal is also available as a 300 mg/5 mL (60mg/mL) oral suspension. Oxcarbazepine is 10,11-Dihydro-10-oxo-5H-dibenz[b,f]azepi... |
| Active Ingredient | Oxcarbazepine |
| Dosage Form | Tablet; Suspension |
| Route | Oral |
| Strength | 150mg; 600mg; 300mg; 300mg/5ml |
| Market Status | Prescription |
| Company | Novartis |
Oxcarbazepine is indicated for monotherapeutic or adjunctive therapeutic use in the treatment of partial seizures in adults and children ages 4 to 16 with epilepsy. /Included in US product labeling/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 2234
/EXPL THER:/ ... Oxcarbazepine /was compared/ with acamprosate in relapse prevention in recently withdrawn alcohol-dependent patients. /They/ investigated the efficacy and safety of oxcarbazepin (vs acamprosate) by conducting a 24-week randomized, parallel-group, open-label, clinical trial on 30 acutely detoxified alcoholic patients. Survival analyses (Kaplan-Meier) were performed to look for evidence of a longer "survival" of patients receiving oxcarbazepine. ... After withdrawal, time to severe relapse and time to first consumption of any ethanol by oxcarbazepin patients were not longer than for acamprosate patients. Abstinent patients in both study groups showed a significantly lower obsessive compulsive drinking scale-German version (OCDS-G) than relapsed patients. No undesired effects occurred when oxcarbazepin patients consumed alcohol. ... It is noteworthy that oxcarbazepine is well tolerated, even when alcohol is on board. ...
PMID:16573580 Croissant B et al; Alcohol Clin Exp Res 30 (4): 630-5 (2006)
/EXPL THER:/ ... Data related to 150 patients harboring supratentorial brain gliomas with the aim to assess the efficacy of oxcarbazepine in preventing the occurrence or the recurrence of early postoperative seizures and its tolerability when it is rapidly titrated /was analyzed/. Only four patients (2.7%) experienced seizures within the first week after surgery. Patients did not report disturbances during the titration phase. Regarding adverse events in the first week, six patients (4%) showed minor skin rash. Persistent symptomatic hyponatremia never occurred. ... Oxcarbazepine can be a good alternative to traditional antiepileptic agents in the prevention of perioperative seizures being efficacy, ease of use (rapid titration in 3 days, not requiring close plasma concentration monitoring) and good tolerability (no major side effects during titration and during the first postoperative week) the key factors. Moreover, oxcarbazepine can be a valid choice when long-term therapy is required because of the low interaction with other drugs and the low hematological side effects.
PMID:16944312 Mauro AM et al; J Neurooncol 81 (3): 279-85 (2007)
Multiorgan hypersensitivity reactions occurring days to weeks or months (range 4-60 days) after initiation of oxcarbazepine therapy have been reported in adults and pediatric patients. Although these reactions have been reported rarely, many of these patients required hospitalization, and some reactions were considered life-threatening. Manifestations may include (but are not limited to) fever, rash, lymphadenopathy, hepatitis, abnormal liver function test results, eosinophilia, thrombocytopenia, neutropenia, pruritus, nephritis, oliguria, hepatorenal syndrome, arthralgia, and asthenia.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 2245
While severe hyponatremia is reported to be more frequent in adults treated with oxcarbazepine than with carbamazepine, there is not sufficient data about the incidence of hyponatremia in childhood during treatment with oxcarbazepine. ... Changes in serum electrolyte balance in 75 children with epilepsy before and during treatment with oxcarbazepine and after replacing carbamazepine therapy with oxcarbazepine therapy /were evaluate/. All patients had normal sodium serum levels at the onset of oxcarbazepine. During treatment with oxcarbazepine ... hyponatremia (Na +< 135 mmol/L) without clinical symptoms /were found/ in 26.6 % of the children (n = 20), /and/ sodium levels below 125 mmol/L were observed in 2 children (2.6 %). Clinically relevant hyponatremia occurred in one girl only (1.3 %). In a subgroup of 27 children, in whom carbamazepine was directly replaced with oxcarbazepine, hyponatremia without symptoms was found in one child under carbamazepine (3.7 %) and in six children under oxcarbazepine (22.2 %). Dosage of oxcarbazepine, serum levels of the active metabolite of oxcarbazepine, antiepileptic comedication or patients' age and gender were of no predictive value for the development of hyponatremia. ...
PMID:12571784 Holtmann M et al; Neuropediatrics 33 (6): 298-300 (2002)
Adverse effects occurring in 5% or more of patients and more frequently than placebo include dizziness, somnolence, diplopia, fatigue, nausea, vomiting, ataxia, abnormal vision, abdominal pain, tremor, dyspepsia, abnormal gait.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 2246
Serious dermatologic reactions, including Stevens-Johnson syndrome and toxic epidermal necrolysis, have been reported in adults and children receiving oxcarbazepine; reactions have been life-threatening, have required hospitalization, and rarely have been fatal. The incidence of Stevens-Johnson syndrome and toxic epidermal necrolysis reported in patients receiving oxcarbazepine exceeds the rate in the general population by threefold to tenfold. The median time to onset of these reactions was 19 days. Recurrence of serious dermatologic reactions following rechallenge with oxcarbazepine has occurred.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 2245
For more Drug Warnings (Complete) data for OXCARBAZEPINE (11 total), please visit the HSDB record page.
In the United States, oxcarbazepine is indicated as monotherapy in the treatment of partial-onset seizures in patients 4 years of age and older, and as adjunctive therapy in the treatment of partial-onset seizures in patients 2 years of age and older. In Canada, oxcarbazepine is indicated for use as monotherapy or adjunctive therapy in the treatment of partial-onset seizures in patients 6 years of age and older.
Oxcarbazepine is an anticonvulsant drug that reduces the incidence of seizures in epilepsy by inhibiting abnormal electrical activity in the brain. There have been rare reports of oxcarbazepine resulting in the development of hematologic abnormalities, including agranulocytosis and aplastic anemia. Patients should be undergo frequent laboratory testing and should be monitored closely for signs and symptoms of blood dyscrasias. Oxcarbazepine has also been associated with the development of dermatologic reactions which can progress from a simple rash to potentially fatal reactions such as toxic epidermal necrolysis (TEN) or Stevens-Johnson Syndrome (SJS). Patients with the HLA-A*3101 and/or HLA-B*1502 alleles may be at higher risk of this reaction. Oxcarbazepine should be discontinued at the first sign of a drug-induced skin reaction.
Anticonvulsants
Drugs used to prevent SEIZURES or reduce their severity. (See all compounds classified as Anticonvulsants.)
Cytochrome P-450 CYP3A Inducers
Drugs and compounds that induce the synthesis of CYTOCHROME P-450 CYP3A. (See all compounds classified as Cytochrome P-450 CYP3A Inducers.)
Voltage-Gated Sodium Channel Blockers
A class of drugs that inhibit the activation of VOLTAGE-GATED SODIUM CHANNELS. (See all compounds classified as Voltage-Gated Sodium Channel Blockers.)
N - Nervous system
N03 - Antiepileptics
N03A - Antiepileptics
N03AF - Carboxamide derivatives
N03AF02 - Oxcarbazepine
Absorption
Oxcarbazepine is completely absorbed following oral administration. A single 600mg dose of oxcarbazepine resulted in an MHD Cmax of 34 mol/L and a median Tmax of 4.5 hours. When administered twice daily, steady-state levels of MHD are attained within 2-3 days. The rate and extent of absorption of oxcarbazepine is not affected by food intake.
Route of Elimination
Following oral administration, more than 95% of the administered dose of oxcarbazepine is found in the urine. Of this, approximately 49% is MHD glucuronide metabolites, 27% is unchanged MHD, 3% is inactive DHD metabolites, 13% is conjugated oxcarbazepine, and less than 1% is unchanged parent drug. Fecal elimination accounts for only 4% of the administered dose.
Volume of Distribution
The apparent volume of distribution of oxcarbazepine is 49 L. The apparent volumes of distribution of (S)- and (R)-MHD were found to be 23.6 L and 31.7 L, respectively.
Clearance
Plasma clearance of oxcarbazepine has been estimated to be approximately 84.9 L/h, whereas plasma clearance of its active metabolite, MHD, was estimated to be 2.0 L/h. Rapid metabolic clearance appears to be the main pathway for oxcarbazepine, while clearance of its metabolites occurs mainly via renal excretion.
Oxcarbazepine is completely absorbed. Food does not alter the rate and extent of absorption of oxcarbazepine.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 2235
Both oxcarbazepine and its active 10-monohydroxy metabolite (MHD) are distributed into milk in humans.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 2246
Elimination: Renal: greater than 95%, with more than 99% of the dose excreted in the form of metabolites. Fecal: less than 4%.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 2235
Oxcarbazepine is an antiepileptic drug with a chemical structure similar to carbamazepine, but with different metabolism. Oxcarbazepine is rapidly reduced to 10,11-dihydro-10-hydroxy-carbazepine (monohydroxy derivative, MHD), the clinically relevant metabolite of oxcarbazepine. MHD has (S)-(+)- and the (R)-(-)-enantiomer, but the pharmacokinetics of the racemate are usually reported. The bioavailability of the oral formulation of oxcarbazepine is high (>95%). It is rapidly absorbed after oral administration, reaching peak concentrations within about 1-3 hours after a single dose, whereas the peak of MHD occurs within 4-12 hours. At steady state, the peak of MHD occurs about 2-4 hours after drug intake. The plasma protein binding of MHD is about 40%. Cerebrospinal fluid concentrations of MHD are in the same range as unbound plasma concentrations of MHD. Oxcarbazepine can be transferred significantly through the placenta in humans. Oxcarbazepine and MHD exhibit linear pharmaco-kinetics and no autoinduction occurs. ...
PMID:12959634 May TW et al; Clin Pharmacokinet 42 (12): 1023-42 (2003)
For more Absorption, Distribution and Excretion (Complete) data for OXCARBAZEPINE (9 total), please visit the HSDB record page.
Oxcarbazepine is rapidly and extensively metabolized to its primary metabolite, MHD, which is responsible for the bulk of its anti-epileptic activity and exists in much higher concentrations in the plasma than the parent drug. MHD is formed via reduction by several members of the aldo-keto reductase family of cytosolic liver enzymes and exists as a racemate in plasma in an approximate ratio of 80% (S)-MHD to 20% (R)-MHD. MHD is further metabolized to glucuronide conjugate metabolites for excretion, and small amounts are oxidized to 10-,11-dihydro-10,11-dihydroxycarbamazepine (DHD) which is pharmacologically inactive. Only 10% of an administered dose of oxcarbazepine will remain as either the parent drug or glucuronide conjugates of the parent drug.
Oxcarbazepine is rapidly reduced by cytosolic enzymes in the liver to its 10-monohydroxy metabolite, MHD, which is primarily responsible for the pharmacological effect of Trileptal. MHD is metabolized further by conjugation with glucuronic acid. Minor amounts (4% of the dose) are oxidized to the pharmacologically inactive 10,11-dihydroxy metabolite (DHD). Oxcarbazepine is cleared from the body mostly in the form of metabolites which are predominantly excreted by the kidneys. More than 95% of the dose appears in the urine, with less than 1% as unchanged oxcarbazepine. Fecal excretion accounts for less than 4% of the administered dose. Approximately 80% of the dose is excreted in the urine either as glucuronides of MHD (49%) or as unchanged MHD (27%); the inactive DHD accounts for approximately 3% and conjugates of MHD and oxcarbazepine account for 13% of the dose.
Physicians Desk Reference 61st ed, Thomson PDR, Montvale, NJ 2007., p. 2300
The disposition of the new anti-epileptic agent oxcarbazepine (10,11-dihydro-10-oxo-5H-dibenz[b,f]azepine-5-carboxamide) has been studied in two healthy volunteers following an oral 400 mg dose of (14)C-labelled drug. The dose was excreted almost completely in the urine (94.6 and 97.1%) within six days. Fecal excretion amounted to 4.3 and 1.9% of the dose in the two subjects. In the 0-6 days urine samples the biotransformation products have been isolated and identified. 10,11-Dihydro-10-hydroxycarbamazepine (GP 47,779) and its two diastereoisomeric O-glucuronides were found as main metabolites. Taken together, they accounted for 79% of urinary (14)C. Unchanged oxcarbazepine, and its sulfate and glucuronide conjugates were isolated in smaller amounts only (13%). Other minor metabolites were the trans- and cis-isomers of 10,11-dihydro-10,11-dihydroxy-carbamazepine (approximately 4%), and a phenolic derivative of GP 47,779 (less than 1%). The biotransformation of oxcarbazepine proceeds mainly by reduction to GP 47,779, and subsequent conjugation with glucuronic acid. Reduction is stereospecific, favoring the S-configuration of GP 47,779. Direct conjugation of oxcarbazepine, in the enol form, is a minor pathway. Oxidative reactions are unimportant.
PMID:3765657 Schutz H et al; Xenobiotica 16 (8): 769-78 (1986)
... The interaction potential of oxcarbazepine is relatively low. However, enzyme-inducing antiepileptic drugs such as phenytoin, phenobarbital or carbamazepine can reduce slightly the concentrations of 10,11-dihydro-10-hydroxy-carbazepine (monohydroxy derivative, MHD). Verapamil may moderately decrease MHD concentrations, but this effect is probably without clinical relevance. The influence of oxcarbazepine on other antiepileptic drugs is not clinically relevant in most cases. However, oxcarbazepine appears to increase concentrations of phenytoin and to decrease trough concentrations of lamotrigine and topiramate. Oxcarbazepine lowers concentrations of ethinylestradiol and levonorgestrel, and women treated with oxcarbazepine should consider additional contraceptive measures. Due to the absent or lower enzyme-inducing effect of oxcarbazepine, switching from carbamazepine to oxcarbazepine can result in increased serum concentrations of comedication, sometimes associated with adverse effects. ...
PMID:12959634 May TW et al; Clin Pharmacokinet 42 (12): 1023-42 (2003)
The plasma half-life of oxcarbazepine is approximately 2 hours and the plasma half-life of MHD is approximately 9 hours.
Oxcarbazepine: 2 hours. 10-Monohydroxy metabolite: 9 hours. Note: In patients with renal function impairment with a creatinine clearance < 30 mL/minute, the half life of 10 monohydroxy metabolite is prolonged to 10 hours ...
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 2235
... Elimination half-lives in healthy volunteers are 1-5 hours for oxcarbazepine and 7-20 hours for 10,11-dihydro-10-hydroxy-carbazepine (monohydroxy derivative, MHD). Longer and shorter elimination half-lives have been reported in elderly volunteers and children, respectively. ...
PMID:12959634 May TW et al; Clin Pharmacokinet 42 (12): 1023-42 (2003)
The exact mechanism through which oxcarbazepine and its active metaoblite, MHD, exert their anti-epileptic effects is unclear, but is thought to primarily involve the blockade of voltage-gated sodium channels. The opening and closing of sodium channels allows for the propagation of action potentials along neurons - in epilepsy, these action potentials can occur in excess of that required for normal function, and the repetitive and pathological firing of these action potentials leads to seizure activity. Both oxcarbazepine and MHD are thought to inhibit seizure activity by binding to the inactive state of voltage-gated sodium channels, thus prolonging the period in which the receptor is unavailable for action potential propagation. This helps to stabilize hyperexcited neuronal membranes, inhibit repetitive neuron firing, and prevent the spread of seizure activity within the CNS without affecting normal neuronal transmission. Increased potassium conductance and modulation of voltage-activated calcium channels is also thought to play a role in the anti-seizure activity of oxcarbazepine. Inhibition of glutamatergic activity was thought to contribute to oxcarbazepine's activity, but this effect could not be replicated _in vivo_.
The pharmacological activity of Trileptal (oxcarbazepine) is primarily exerted through the 10-monohydroxy metabolite (MHD) of oxcarbazepine. The precise mechanism by which oxcarbazepine and MHD exert their antiseizure effect is unknown; however, in vitro electrophysiological studies indicate that they produce blockade of voltage-sensitive sodium channels, resulting in stabilization of hyperexcited neural membranes, inhibition of repetitive neuronal firing, and diminution of propagation of synaptic impulses. These actions are thought to be important in the prevention of seizure spread in the intact brain. In addition, increased potassium conductance and modulation of high-voltage activated calcium channels may contribute to the anticonvulsant effects of the drug. No significant interactions of oxcarbazepine or MHD with brain neurotransmitter or modulator receptor sites have been demonstrated.
Physicians Desk Reference 61st ed, Thomson PDR, Montvale, NJ 2007., p. 2300

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
99
PharmaCompass offers a list of Oxcarbazepine API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Oxcarbazepine manufacturer or Oxcarbazepine supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Oxcarbazepine manufacturer or Oxcarbazepine supplier.
PharmaCompass also assists you with knowing the Oxcarbazepine API Price utilized in the formulation of products. Oxcarbazepine API Price is not always fixed or binding as the Oxcarbazepine Price is obtained through a variety of data sources. The Oxcarbazepine Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Oxetol manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Oxetol, including repackagers and relabelers. The FDA regulates Oxetol manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Oxetol API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Oxetol manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Oxetol supplier is an individual or a company that provides Oxetol active pharmaceutical ingredient (API) or Oxetol finished formulations upon request. The Oxetol suppliers may include Oxetol API manufacturers, exporters, distributors and traders.
click here to find a list of Oxetol suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Oxetol DMF (Drug Master File) is a document detailing the whole manufacturing process of Oxetol active pharmaceutical ingredient (API) in detail. Different forms of Oxetol DMFs exist exist since differing nations have different regulations, such as Oxetol USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Oxetol DMF submitted to regulatory agencies in the US is known as a USDMF. Oxetol USDMF includes data on Oxetol's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Oxetol USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Oxetol suppliers with USDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Oxetol Drug Master File in Korea (Oxetol KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Oxetol. The MFDS reviews the Oxetol KDMF as part of the drug registration process and uses the information provided in the Oxetol KDMF to evaluate the safety and efficacy of the drug.
After submitting a Oxetol KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Oxetol API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Oxetol suppliers with KDMF on PharmaCompass.
A Oxetol CEP of the European Pharmacopoeia monograph is often referred to as a Oxetol Certificate of Suitability (COS). The purpose of a Oxetol CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Oxetol EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Oxetol to their clients by showing that a Oxetol CEP has been issued for it. The manufacturer submits a Oxetol CEP (COS) as part of the market authorization procedure, and it takes on the role of a Oxetol CEP holder for the record. Additionally, the data presented in the Oxetol CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Oxetol DMF.
A Oxetol CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Oxetol CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Oxetol suppliers with CEP (COS) on PharmaCompass.
A Oxetol written confirmation (Oxetol WC) is an official document issued by a regulatory agency to a Oxetol manufacturer, verifying that the manufacturing facility of a Oxetol active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Oxetol APIs or Oxetol finished pharmaceutical products to another nation, regulatory agencies frequently require a Oxetol WC (written confirmation) as part of the regulatory process.
click here to find a list of Oxetol suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Oxetol as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Oxetol API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Oxetol as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Oxetol and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Oxetol NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Oxetol suppliers with NDC on PharmaCompass.
Oxetol Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Oxetol GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Oxetol GMP manufacturer or Oxetol GMP API supplier for your needs.
A Oxetol CoA (Certificate of Analysis) is a formal document that attests to Oxetol's compliance with Oxetol specifications and serves as a tool for batch-level quality control.
Oxetol CoA mostly includes findings from lab analyses of a specific batch. For each Oxetol CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Oxetol may be tested according to a variety of international standards, such as European Pharmacopoeia (Oxetol EP), Oxetol JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Oxetol USP).

