Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
FDF Dossiers
0
FDA Orange Book
0
Europe
0
Canada
0
Australia
0
South Africa
0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
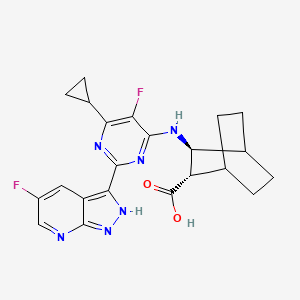

1. 2200336-20-3
2. Onradivir [inn]
3. Cze6g5g466
4. (2s,3s)-3-((6-cyclopropyl-5-fluoro-2-(5-fluoro-1h-pyrazolo(3,4-b)pyridin-3-yl)pyrimidin-4-yl)amino)bicyclo(2.2.2)octane-2-carboxylic Acid
5. (2s,3s)-3-((6-cyclopropyl-5-fluoro-2-(5-fluoro-1h-pyrazolo(3,4-b)pyridin-3-yl)-4-pyrimidinyl)amino)bicyclo(2.2.2)octane-2-carboxylic Acid
6. Bicyclo(2.2.2)octane-2-carboxylic Acid, 3-((6-cyclopropyl-5-fluoro-2-(5-fluoro-1h-pyrazolo(3,4-b)pyridin-3-yl)-4-pyrimidinyl)amino)-, (2s,3s)-
7. Refchem:168306
8. Unii-cze6g5g466
9. Chembl5095075
10. Schembl21128398
11. Glxc-26953
12. Ex-a12514
13. (2s,3s)-3-[[6-cyclopropyl-5-fluoro-2-(5-fluoro-2h-pyrazolo[3,4-b]pyridin-3-yl)pyrimidin-4-yl]amino]bicyclo[2.2.2]octane-2-carboxylic Acid
14. Da-66347
15. Hy-145586
16. Cs-0376485
| Molecular Weight | 440.4 g/mol |
|---|---|
| Molecular Formula | C22H22F2N6O2 |
| XLogP3 | 3.9 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 5 |
| Exact Mass | Da |
| Monoisotopic Mass | Da |
| Topological Polar Surface Area | 117 |
| Heavy Atom Count | 32 |
| Formal Charge | 0 |
| Complexity | 721 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
ABOUT THIS PAGE

