
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
Europe
0
Canada
0
Australia
0
South Africa
0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
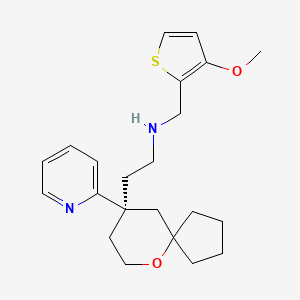

1. ((3-methoxythiophen-2-yl)methyl)((2-(9-(pyridin-2-yl)-6-oxaspiro(4.5)decan-9-yl)ethyl))amine
2. Olinvyk
3. Trv-130
4. Trv130
1. Trv130
2. 1401028-24-7
3. Trv-130
4. Olinvyk
5. Oliceridine [usan]
6. Mcn858tcp0
7. N-[(3-methoxythiophen-2-yl)methyl]-2-[(9r)-9-pyridin-2-yl-6-oxaspiro[4.5]decan-9-yl]ethanamine
8. Olinvo
9. [(3-methoxythiophen-2-yl)methyl]({2-[(9r)-9-(pyridin-2-yl)-6-oxaspiro[4.5]decan-9-yl]ethyl})amine
10. 6-oxaspiro(4.5)decane-9-ethanamine, N-((3-methoxy-2-thienyl)methyl)-9-(2-pyridinyl)-, (9r)-
11. 6-oxaspiro[4.5]decane-9-ethanamine, N-[(3-methoxy-2-thienyl)methyl]-9-(2-pyridinyl)-, (9r)-
12. N-((3-methoxythiophen-2-yl)methyl)-2-((9r)-9-(pyridin-2-yl)-6-oxaspiro(4.5)decan-9-yl)ethanamine
13. N-[(3-methoxythiophen-2-yl)methyl]-2-[(9r)-9-(pyridin-2-yl)-6-oxaspiro[4.5]decan-9-yl]ethan-1-amine
14. Trv 130
15. Unii-mcn858tcp0
16. Trv130a
17. Olinvyk (tn)
18. Oliceridine [mi]
19. Oliceridine; Trv-130
20. Oliceridine [inn]
21. Oliceridine (usan/inn)
22. Oliceridine [who-dd]
23. Gtpl7334
24. Chembl2443262
25. Schembl12542370
26. Oliceridine [orange Book]
27. Dtxsid201031292
28. Bcp14196
29. Bgc02824
30. Ex-a2170
31. Bdbm50493818
32. Zinc96940334
33. Akos030526482
34. Oliceridine;olinvo;trv 130;trv130
35. Cs-3571
36. Db14881
37. Ncgc00390659-01
38. Ac-35966
39. Hy-16655
40. A14337
41. D11214
42. A935006
43. Q17142898
44. (9r)-n-[(3-methoxy-2-thienyl)methyl]-9-(2-pyridinyl)-6-oxaspiro[4.5]decane-9-ethanamine Hydrochlorid
45. (r)-n-((3-methoxythiophen-2-yl)methyl)-2-(9-(pyridin-2-yl)-6-oxaspiro[4.5]decan-9-yl)ethanamine
46. [(3-methoxythiophen-2-yl)methyl]({2-[(9r)-9-(pyridine-2-yl)-6-oxaspiro[4.5]decan-9-yl]ethyl})amine
47. Wh2
| Molecular Weight | 386.6 g/mol |
|---|---|
| Molecular Formula | C22H30N2O2S |
| XLogP3 | 3.5 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 7 |
| Exact Mass | 386.20279938 g/mol |
| Monoisotopic Mass | 386.20279938 g/mol |
| Topological Polar Surface Area | 71.6 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 471 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Oliceridine is indicated for the management of acute pain in adults severe enough to require intravenous opioid analgesics and for whom no acceptable alternative treatments exist.
Oliceridine is a biased -opioid receptor agonist that acts through downstream signalling pathways to exert antinociceptive analgesia in patients experience severe acute pain. Results from multiple clinical studies and simulation data demonstrate that oliceridine exerts significant analgesic benefits within 5-20 minutes following administration but dissipates quickly with a half-life between one and three hours. Despite an improved adverse effect profile over conventional opioids, oliceridine carries important clinical warnings. Oliceridine has the potential to cause severe respiratory depression, especially in patients who are elderly, cachectic, debilitated, or who otherwise have chronically impaired pulmonary function. In addition, severe respiratory depression or sedation may occur in patients with increased intracranial pressure, head injury, brain tumour, or impaired consciousness. Patients with adrenal insufficiency or severe hypotension may require treatment alterations or discontinuation. Finally, oliceridine has been demonstrated to prolong the QTc interval and has not been properly evaluated beyond a maximum daily dose of 27 mg; it is recommended not to exceed 27 mg per day.
N - Nervous system
N02 - Analgesics
N02A - Opioids
N02AX - Other opioids
N02AX07 - Oliceridine
Absorption
Oliceridine administered as a single intravenous injection of 1.5, 3, or 4.5 mg in healthy male volunteers had a corresponding Cmax of 47, 76, and 119 ng/mL and a corresponding AUC0-24 of 43, 82, and 122 ng\*h/mL. Simulations of single doses of oliceridine between 1-3 mg suggest that the expected median Cmax is between 43 and 130 ng/mL while the expected median AUC is between 22 and 70 ng\*h/mL.
Route of Elimination
Approximately 70% of oliceridine is eliminated via the renal route, of which only 0.97-6.75% of an initial dose is recovered unchanged. The remaining 30% is eliminated in feces.
Volume of Distribution
Oliceridine has a mean steady-state volume of distribution of 90-120 L.
Clearance
Healthy volunteers given doses of oliceridine between 0.15 and 7 mg had mean clearance rates between 34 and 59.6 L/h.
Oliceridine is primarily metabolized hepatically by CYP3A4 and CYP2D6 _in vitro_, with minor contributions from CYP2C9 and CYP2C19. None of oliceridine's metabolites are known to be active. Metabolic pathways include N-dealkylation, glucuronidation, and dehydrogenation.
Oliceridine has a half-life of 1.3-3 hours while its metabolites, none of which are known to be active, have a substantially longer half-life of 44 hours.
Pain perception follows a complex pathway initiated in primary sensory neurons, subsequently transmitted to the spinal cord dorsal horn and through ascending axons to multiple regions within the thalamus, brainstem, and midbrain, and finally relayed through descending signals that either inhibit or facilitate the nociceptive signalling. Opioid receptors are seven-transmembrane G-protein-coupled receptors (GPCRs) that can be divided into , , , and opioid-like-1 (ORL1) subtypes,. However, the -opioid receptor is predominantly targeted by and is responsible for the effects of traditional opioids. GPCRs in the inactive state are bound intracellularly by a complex consisting of a G, , and subunit together with guanosine diphosphate (GDP). Activation of the GPCR through extracellular agonist binding catalyzes the replacement of GDP with guanosine triphosphate (GTP), dissociation of both G-GTP and a heterodimer, and subsequent downstream effects. In the case of the -opioid receptor, the G-GTP directly interacts with the potassium channel Kir3 while the dissociated G subunit directly binds to and occludes the pore of P/Q-, N-, and L-type Ca2+ channels. Furthermore, opioid receptor activation inhibits adenylyl cyclase, which in turn reduces cAMP-dependent Ca2+ influx. By altering membrane ion conductivity, these effects modulate nociceptive signalling and produce an analgesic effect. In addition to the G-protein pathway, -opioid receptor activation can also result in downstream signalling through -arrestin, which results in receptor internalization and is associated with negative effects of opioid use including respiratory depression, gastrointestinal effects, and desensitization/tolerance. Oliceridine acts as a "biased agonist" at the -opioid receptor by preferentially activating the G-protein pathway with minimal receptor phosphorylation and recruitment of -arrestin. Competetive binding assays and structural modelling suggest that the binding site for oliceridine on the -opioid receptor is the same as for classical opioids. However, molecular modelling supports a model whereby oliceridine binding induces a different intracellular conformation of the -opioid receptor, specifically due to a lack of coupling with transmembrane helix six, which confers the specificity for G-protein over -arrestin interaction. Numerous _in vitro_, _in vivo_, and clinical studies support the view that this biased agonism results in comparable analgesia compared with traditional opioids at a comparable or decreased risk of opioid-related adverse effects such as constipation and respiratory depression.
Market Place

Patents & EXCLUSIVITIES
ABOUT THIS PAGE
54
PharmaCompass offers a list of Oliceridine API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Oliceridine manufacturer or Oliceridine supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Oliceridine manufacturer or Oliceridine supplier.
PharmaCompass also assists you with knowing the Oliceridine API Price utilized in the formulation of products. Oliceridine API Price is not always fixed or binding as the Oliceridine Price is obtained through a variety of data sources. The Oliceridine Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Oliceridine manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Oliceridine, including repackagers and relabelers. The FDA regulates Oliceridine manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Oliceridine API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Oliceridine manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Oliceridine supplier is an individual or a company that provides Oliceridine active pharmaceutical ingredient (API) or Oliceridine finished formulations upon request. The Oliceridine suppliers may include Oliceridine API manufacturers, exporters, distributors and traders.
click here to find a list of Oliceridine suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
Oliceridine Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Oliceridine GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Oliceridine GMP manufacturer or Oliceridine GMP API supplier for your needs.
A Oliceridine CoA (Certificate of Analysis) is a formal document that attests to Oliceridine's compliance with Oliceridine specifications and serves as a tool for batch-level quality control.
Oliceridine CoA mostly includes findings from lab analyses of a specific batch. For each Oliceridine CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Oliceridine may be tested according to a variety of international standards, such as European Pharmacopoeia (Oliceridine EP), Oliceridine JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Oliceridine USP).

