
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
Europe
0
Australia
0
South Africa
0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
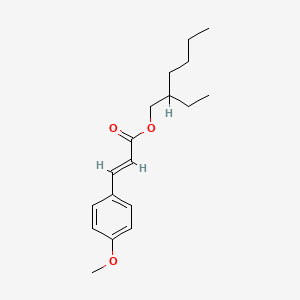

1. 2-ethylhexyl-4-methoxycinnamate
2. 2-ethylhexyl-p-methoxycinnamate
3. Escalol 557
4. Heliopan New
5. Octyl-methoxycinnamate
6. Octylmethoxycinnamate
7. Omc Cinnamate
8. Parsol Mcx
1. 5466-77-3
2. 83834-59-7
3. 2-ethylhexyl 4-methoxycinnamate
4. Parsol Mcx
5. Parsol Mox
6. Parsol
7. 2-ethylhexyl P-methoxycinnamate
8. 2-ethylhexyl-p-methoxycinnamate
9. 4-methoxycinnamic Acid 2-ethylhexyl Ester
10. 2-ethylhexyl Trans-4-methoxycinnamate
11. Octyl Methoxycinnamate
12. Escalol 557
13. 2-ethylhexyl 3-(4-methoxyphenyl)acrylate
14. Octyl-methoxycinnamate
15. Ethylhexyl Methoxycinnamate
16. (e)-2-ethylhexyl 3-(4-methoxyphenyl)acrylate
17. Neo Heliopan Av
18. 2-ethylhexyl (e)-3-(4-methoxyphenyl)prop-2-enoate
19. 2-ethylhexyl-4-methoxycinnamate
20. Sunscreen Av
21. Uvinul Mc 80
22. Octyl P-methoxycinnamate
23. Ethylhexyl P-methoxycinnamate
24. Octyl 4-methoxycinnamic Acid
25. 2-ethylhexyl
26. Trans
27. -4-methoxycinnamate
28. 4y5p7mud51
29. Chebi:88667
30. 2-propenoic Acid, 3-(4-methoxyphenyl)-, 2-ethylhexyl Ester, (2e)-
31. 2-ethylhexyl Methoxycinnamate
32. Octylmethoxycinnamate
33. Uvinul Mc80
34. 2-propenoic Acid, 3-(4-methoxyphenyl)-, 2-ethylhexyl Ester
35. Nsc-26466
36. Nsc 26466
37. Ocinoxate
38. Jeescreen Omc
39. Octinoxate [usan]
40. Solarom Omc
41. Parsol Mcx-sa
42. Escalol 557nb
43. Uvinul Mc 80n
44. Escalol 557t
45. Heliopan New
46. Uvinul Mc 90
47. Eusolex Uv-pearls Omc
48. Sun Caps 664
49. Unii-4y5p7mud51
50. Ncgc00160623-01
51. Parsol (tn)
52. Uvinult Mc 80 N
53. 2-ethylhexyl 3-(4-methoxyphenyl)-2-propenoate
54. Neo Heliopan, Type Av
55. Octinoxate (usp/inn)
56. Octinoxate [inn]
57. Octinoxate [hsdb]
58. Octinoxate [vandf]
59. Ec 629-661-9
60. Octinoxate [mart.]
61. Dsstox_cid_28119
62. Dsstox_rid_82199
63. Octinoxate [usp-rs]
64. Octinoxate [who-dd]
65. Dsstox_gsid_47205
66. Schembl15609
67. Mls004773966
68. Chembl1200608
69. Dtxsid9047205
70. Octinoxate [orange Book]
71. 3-(4-methoxyphenyl)-2-propenoic Acid 2-ethylhexyl Ester
72. Octinoxate [usp Monograph]
73. 4-methoxycinnamic Acid Octyl Ester
74. Octyl Methoxycinnamate [mi]
75. Hy-b1234
76. Nsc26466
77. P-methoxyzimtsaure-2-ethylhexylester
78. Tox21_302576
79. Mfcd00072582
80. S5320
81. Octyl Methoxycinnamate [vandf]
82. Akos015838519
83. 2-ethylhexyl 4-methoxycinnamate, 98%
84. Ccg-267384
85. Cs-4732
86. Db09496
87. Ncgc00160623-02
88. Ncgc00160623-03
89. Ncgc00181309-01
90. Ncgc00256897-01
91. As-11708
92. Ethylhexyl Methoxycinnamate [inci]
93. Shade Uvaguard Component Octinoxate
94. Smr001550370
95. Cas-83834-59-7
96. 2-ethylhexyl Trans-4-methoxycinnamate, 98%
97. 4-methoxycinnamicacid2-ethylhexylester
98. Ethylhexyl P-methoxycinnamate [vandf]
99. M1082
100. Octinoxate Component Of Shade Uvaguard
101. (e)-2-ethylhexyl3-(4-methoxyphenyl)acrylate
102. D05225
103. D95744
104. P-methoxycinnamic Acid 2-ethylhexyl Ester
105. A840663
106. Q739648
107. Sr-01000883955
108. 2-ethylhexyl 4-methoxycinnamate, Analytical Standard
109. Sr-01000883955-1
110. 3-(4-methoxy-phenyl)-acrylic Acid 2-ethyl-hexyl Ester
111. C118580000
112. Octinoxate, Pharmaceutical Secondary Standard; Traceable To Usp
113. Octinoxate, United States Pharmacopeia (usp) Reference Standard
114. 2-propenoic Acid, 3-(4-methoxyphenyl)-, 2-ethylhexyl Ester, (e)-
115. Octinoxate, Pharmaceutical Secondary Standard; Certified Reference Material
116. 2-ethylhexyl Trans-4-methoxycinnamate, 98%, Contains 500-1000 Ppm Bht As Stabilizer
117. Octyl Methoxycinnamate 7.5%, Octinoxate, Octyl Methoxycinnamate, Octyl Methoxycinnamate (2-ethylhexyl Trans-4-methoxycinnamate)
| Molecular Weight | 290.4 g/mol |
|---|---|
| Molecular Formula | C18H26O3 |
| XLogP3 | 5.3 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 10 |
| Exact Mass | 290.18819469 g/mol |
| Monoisotopic Mass | 290.18819469 g/mol |
| Topological Polar Surface Area | 35.5 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 304 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Ultraviolet /UVB/ screen
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. Cambridge, UK: Royal Society of Chemistry, 2013., p. 1259
/The authors/ tested the sun protection factor of a hydroquinone formulation (Lustra-Ultra, TaroPharma, Hawthorne, NY) containing avobenzone 3%, and octinoxate 7.5% according to the FDA Sunscreen Monograph on 20 volunteer subjects. We also determined the UVR absorbance spectrum of the preparation. ... The mean sun protection factor (SPF) of 21.7 satisfied labeling requirements for SPF 20. The formulation exhibited strongest photoprotection near the wavelengths of peak sun burning effectiveness in the UVB region and maintains significant UVR absorbance through the entire UVA region. Avobenzone 3% and octinoxate 7.5% provide broad spectrum UV protection. Incorporating these sunscreens into a hydroquinone preparation simplifies the treatment regimen while providing significant photoprotection for patients being treated for dyschromia.
PMID:16673798 Stanfield JW et al; J Drugs Dermatol 5 (4): 321-4 (2006)
Sunscreens capable of inhibiting erythema are assumed to protect against UV-induced carcinogenesis as well. However, the correlation between inflammation and carcinogenesis is uncertain, and the prevention of UV-induced erythema might in fact be biologically irrelevant as an indicator of protection against UV-induced skin cancer. Ultraviolet-B radiation promotes cutaneous immunosuppression by the release of immunoregulatory cytokines and by depletion of Langerhans cells. /The authors/ investigated the ability of two different sunscreens to inhibit UVB-induced expression of epidermal interleukin (IL)-10 and depletion of Langerhans cells. Chemical and physical sunscreens were applied to the forearms of volunteers 15 min prior to 4 minimal erythemal doses of UVB exposure. Suction blisters were induced 24 hr after irradiation, and RNA was extracted from the blister roofs. Reverse transcription polymerase chain reaction was performed using primers for IL-10 and CD1a. A chemical sunscreen containing octyl methoxycinnamate (12 sun protection factor (SPF)) and a physical sunscreen containing zinc oxide (16 SPF) were assayed: UVB-induced IL-10 mRNA expression was nearly totally inhibited by both sunscreens (median protection for chemical and physical sunscreens was 95% and 78%, respectively), whereas UVB-induced Langerhans cell depletion was partially prevented (47% and 50% for chemical and physical sunscreens, respectively). Langerhans cell protection by sunscreens was confirmed by estimation of cell density after ATPase staining. In contrast, both sunscreens effectively prevented the induction of UVB-induced erythema. /The authors/ believe this to be the first demonstration that sunscreens can prevent the induction of cutaneous mediators of immunosuppression, and that the results indicate that the immunoprotection offered by the sunscreens is significantly lower than their ability to prevent erythema.
PMID:10568168 Hochberg M, Enk CD; Photochem Photobiol 70 (5): 766-72 (1999)
Daily use of a sunscreen with a high SPF (greater than 15) on usually exposed skin is recommended for residents of areas of high ... /solar radiation/ who work outdoors or ... /enjoy/ regular outdoor recreation. Daily use of a sunscreen can reduce the cumulative ... /solar/ exposure that causes actinic keratoses and squamous-cell carcinoma.
IARC Working Group on the Evaluation of Cancer-Preventive Agents (2001) Sunscreens (IARC Handbooks of Cancer Prevention, Vol. 5), Lyon, IARC; Unit of Chemoprevention: Cancer-Preventive Effects of Sunscreens.
Sunscreen agents are indicated for the prevention of sunburn. In addition to limiting the skin's exposure to the sun, using sunscreen agents regularly when in the sun may help reduce long-term sun damage such as premature aging of the skin and skin cancer. /Sunscreen agents, topical; Included in US product labeling/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006.
The manufacturers of sunscreen preparations with propellants warn that concentrating and subsequently inhaling the fumes from these preparations may be harmful or fatal. /Propellants/
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013
Because the absorptive characteristics of skin of children younger than 6 months of age may differ from those of adults and because the immaturity of metabolic and excretory pathways of these children may limit their ability to eliminate any percutaneously absorbed sunscreen agent, sunscreen products should be used in children younger than 6 months of age only as directed by a clinician. It is possible that the characteristics of geriatric skin also differ from those of skin in younger adults, but these characteristics and the need for special considerations regarding use of sunscreen preparations in this age group are poorly understood. /Sunscreens/
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013
Little information is available regarding the safety of chronic sunscreen usage, but commercially available physical and chemical sunscreens appear to have a low incidence of adverse effects. Derivatives of PABA, benzophenone, cinnamic acid, and salicylate and 2-phenylbenzimidazole-5-sulfonic acid have caused skin irritation including burning, stinging, pruritus, and erythema on rare occasions. /Sunscreens/
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013
Sunscreens should not be used as a means of extending the duration of solar exposure, such as prolonging sunbathing, and should not be used as a substitute for clothing on usually unexposed sites, such as the trunk and buttocks. /Sunscreens/
IARC Working Group on the Evaluation of Cancer-Preventive Agents (2001) Sunscreens (IARC Handbooks of Cancer Prevention, Vol. 5), Lyon, IARC; Unit of Chemoprevention: Cancer-Preventive Effects of Sunscreens.
For more Drug Warnings (Complete) data for OCTINOXATE (11 total), please visit the HSDB record page.
As an active ingredient in sunscreens and lip balms. Used for protection against damaging effects of sun rays.
Acts as a photoprotective agent that protects the skin by preventing and minimizing the damaging effects of ultraviolet (UV) rays of natural light. The cellular effects of UV irradiation include DNA damage, cell cycle arrest, immunological depression, apoptosis, and transcriptional changes.
Sunscreening Agents
Chemical or physical agents that protect the skin from sunburn and erythema by absorbing or blocking ultraviolet radiation. (See all compounds classified as Sunscreening Agents.)
D - Dermatologicals
D02 - Emollients and protectives
D02B - Protectives against uv-radiation
D02BA - Protectives against uv-radiation for topical use
D02BA02 - Octinoxate
Absorption
Can be systemically absorbed after skin application, being found in the deeper layers of the stratum corneum as well as urine, plasma, and breast milk. The mean maximum plasma concentration detected after application of 2mg/cm2 sunscreen was 7ng/mL in women and 16ng/mL in men.
Route of Elimination
Can be detected in urine in unchanged form.
Naked rat skin. This was studied in a chamber experiment. Most of the material was found in the stripped skin; there was less in the stratum corneum, and least in the chamber. The approximate amounts found in the chamber were: after 6 hrs, 1.13 %; after 16 hrs, 11.4 %; and at 24 hrs 17,9 %. The figures for the horny layer and the strippings combined were, respectively, 31.4 %, 44.4 % and 45.7 % (percentages of applied doses). Solutions of 3% and 20 % of a.i. gave similar results.
European Commission; Reports of the Scientific Committee on Cosmetology (Ninth Series): 2-Ethylhexyl-4-methoxycinnamate (5466-77-3) p. 70 (1999). Available from, as of September 10, 2013: https://ec.europa.eu/health/scientific_committees/consumer_safety/docs/scc_o_9.pdf
Eight healthy volunteers had small amounts of radioactive a.i. applied to the interscapular region. One group of 4 had the material applied under a watch glass; the other 4 had it applied on gauze, with occlusion in one case. Tests for absorption of a.i. were negative except for about 0.2 % in urine. The concentrations used were not stated.
European Commission; Reports of the Scientific Committee on Cosmetology (Ninth Series): 2-Ethylhexyl-4-methoxycinnamate (5466-77-3) p. 70 (1999). Available from, as of September 10, 2013: https://ec.europa.eu/health/scientific_committees/consumer_safety/docs/scc_o_9.pdf
In a preliminary experiment, a capsule containing 100 mg of a.i. was taken orally. ... The cumulative excretion of 4methoxycinnamate in the urine over 24 hours was studied by GC/MS of the methyl ester derivative. (This method would also detect 4-hydroxycinnamic acid). Over 24 hours, 13.2 % of the amount ingested was recovered, equivalent to 21.5 % of the amount that would be expected if the a.i. were completely absorbed. In the main part of the experiment, an o/w cream containing 10 % a.i. was used. Applications of 2 grams of this material (= 200 mg a.i.) were made to the interscapular area of each of 5 male subjects, aged 29 to 46. The area of skin covered was 25x30 cm. After application, the area was covered with 3 layers of gauze, left in place for 12 hours. Blood was taken at times 0, 0.5, 1, 2, 3, 5, 7, and 24 hours. Urine was collected at 0, 1, 2, 3, 4, 5, 6, 7, 12, 24, 48, 72 and 96 hours. The control plasma samples showed a level equivalent to about 10 ng/ml before any application had been made. There was no evidence of any rise in plasma levels during the experiment. The urine showed a "physiological" level of 100 to 300 ng/ml. No significant increase in this amount was found in any sample. The authors conclude that very little, if any, of the compound was absorbed under the conditions of the experiment.
European Commission; Reports of the Scientific Committee on Cosmetology (Ninth Series): 2-Ethylhexyl-4-methoxycinnamate (5466-77-3) p. 70 (1999). Available from, as of September 10, 2013: https://ec.europa.eu/health/scientific_committees/consumer_safety/docs/scc_o_9.pdf
The objective of this study was to determine the influence of vehicles on the penetration of octyl methoxycinnamate (OMC), as a UV absorber, to the stratum corneum by the stripping method. The experimental formulations consisted of a conventional o/w emulsion and multilamellar and small unilamellar liposomes (MLVs and SUVs) containing OMC. MLVs containing OMC were prepared by the fusion method and then converted to SUVs by probe sonication. Various formulations were then applied onto the midvolar forearms of six volunteers at a dose of 2 mg/sq cm. After determined timepoints, the stripping method was conducted whereby 22 tape strips were applied and subsequently divided into different stripping groups. The sunscreen agent was assessed by HPLC while the SPF (sun protection factor) of the formulations was determined in human volunteers in accordance with the Australian standard. Overall the results indicate that skin accumulation of OMC in MLVs was significantly greater than in the o/w emulsion and SUVs. Furthermore, SUV's penetration into the deeper skin layers was significantly greater than MLV's and that of a conventional o/w emulsion. Also, higher amounts of OMC were recovered from the upper layers of the stratum corneum than from the deeper layers in all the formulations tested. Finally, the SPF of the liposomes containing OMC was slightly greater than that of the control lotions at a similar concentration of OMC. In conclusion, the result of this study indicates that an MLV prepared by the fusion method could be a better vehicle for OMC as a sunscreen since it has a slightly better SPF compared to a conventional formulation and more remains in the stratum corneum, reducing its penetration to the deeper layers.
PMID:18841304 Golmohammadzadeh S et al; J Cosmet Sci 59 (5): 385-98 (2008)
For more Absorption, Distribution and Excretion (Complete) data for OCTINOXATE (19 total), please visit the HSDB record page.
Can undergo hepatic metabolism when systematically absorbed. Can be enzymatically degraded by lipases in the stratum corneum where esters undergo hydrolysis. Degrade into photoproducts when exposed to sunlight, which leads to a decrease in UV absorption efficiency.
As a lipophilic substance, the a.i. is very likely to be metabolized; it is known in any case to be hydrolyzed by plasma esterases, although slowly.
European Commission; Reports of the Scientific Committee on Cosmetology (Ninth Series): 2-Ethylhexyl-4-methoxycinnamate (5466-77-3) p. 70 (1999). Available from, as of September 10, 2013: https://ec.europa.eu/health/scientific_committees/consumer_safety/docs/scc_o_9.pdf
Absorbs UV-B (predominantly) and UV-A rays while accumulating in the outermost layer of the epidermis. Like any other photoprotective agents, octinoxate prevents the damage to cells and deoxyribonucleic acid (DNA) by reducing the p53 protein expression following UV exposure and also increases the skin's tolerability to UV rays.
Diminish the penetration of ultraviolet (UV) light through the epidermis by absorbing UV radiation within a specific wavelength range. The amount and wavelength of UV radiation absorbed are affected by the molecular structure of the sunscreen agent. /Sunscreen agents, topical/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006.
Radiation is absorbed by chemical sunscreens when the electron energy level of the drug is raised from its ground state to a higher energy level or excited state. Chromophore groups (C=C, C=O, O-N=O) with loosely held electrons are easily excited by radiation. Compounds which have several chromophore groups in optimal positions have high absorbance over a broad range of wavelengths. Chemical sunscreens are usually agents that absorb not less than 85% of UVB radiation (thus preventing burning) but may permit transmission of UVA radiation (thus allowing tanning). Some sunscreens may absorb wavelengths over a range that is slightly wider or narrower than that of UVB. All PABA derivatives absorb wavelengths of approximately 290-320 nm, benzophenone derivatives absorb wavelengths of approximately 250-360 nm, cinnamic acid derivatives absorb wavelengths of 280-320 nm, and salicylate derivatives and other miscellaneous chemical sunscreens absorb wavelengths of about 270-320 nm.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013
The wavelength to which the skin is maximally sensitive had been accepted for many years to be 296.7 nm; however, recent evidence suggests that the most erythemogenic UVB wavelength may be slightly lower (e.g., somewhere in the range of 292-295 nm). In addition, of the stronger burning wavelengths that reach the earth's surface, most are approximately 310 nm. Therefore, sunscreens that maximally absorb UVB radiation near either of these wavelengths are particularly effective at preventing sunburn. Maximum absorbance occurs at about 290 nm for PABA, at about 295 nm for glyceryl-p-aminobenzoate, and at about 310 nm for the remaining PABA derivatives. Maximum absorbance occurs at 280-290 nm for benzophenone derivatives, at 310 nm for cinnamic acid derivatives with the exception of diethanolamine-p-methoxycinnamate which has its maximum absorbance at 290 nm, and at 300-305 nm for salicylate derivatives and other miscellaneous sunscreens. /Sunscreens/
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
75
PharmaCompass offers a list of Octylmethoxycinnamate API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Octylmethoxycinnamate manufacturer or Octylmethoxycinnamate supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Octylmethoxycinnamate manufacturer or Octylmethoxycinnamate supplier.
PharmaCompass also assists you with knowing the Octylmethoxycinnamate API Price utilized in the formulation of products. Octylmethoxycinnamate API Price is not always fixed or binding as the Octylmethoxycinnamate Price is obtained through a variety of data sources. The Octylmethoxycinnamate Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Octylmethoxycinnamate manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Octylmethoxycinnamate, including repackagers and relabelers. The FDA regulates Octylmethoxycinnamate manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Octylmethoxycinnamate API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Octylmethoxycinnamate supplier is an individual or a company that provides Octylmethoxycinnamate active pharmaceutical ingredient (API) or Octylmethoxycinnamate finished formulations upon request. The Octylmethoxycinnamate suppliers may include Octylmethoxycinnamate API manufacturers, exporters, distributors and traders.
click here to find a list of Octylmethoxycinnamate suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Octylmethoxycinnamate DMF (Drug Master File) is a document detailing the whole manufacturing process of Octylmethoxycinnamate active pharmaceutical ingredient (API) in detail. Different forms of Octylmethoxycinnamate DMFs exist exist since differing nations have different regulations, such as Octylmethoxycinnamate USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Octylmethoxycinnamate DMF submitted to regulatory agencies in the US is known as a USDMF. Octylmethoxycinnamate USDMF includes data on Octylmethoxycinnamate's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Octylmethoxycinnamate USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Octylmethoxycinnamate suppliers with USDMF on PharmaCompass.
Octylmethoxycinnamate Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Octylmethoxycinnamate GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Octylmethoxycinnamate GMP manufacturer or Octylmethoxycinnamate GMP API supplier for your needs.
A Octylmethoxycinnamate CoA (Certificate of Analysis) is a formal document that attests to Octylmethoxycinnamate's compliance with Octylmethoxycinnamate specifications and serves as a tool for batch-level quality control.
Octylmethoxycinnamate CoA mostly includes findings from lab analyses of a specific batch. For each Octylmethoxycinnamate CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Octylmethoxycinnamate may be tested according to a variety of international standards, such as European Pharmacopoeia (Octylmethoxycinnamate EP), Octylmethoxycinnamate JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Octylmethoxycinnamate USP).

