
Synopsis
0
USDMF
0
CEP/COS
0
EU WC
0
KDMF
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
FDF Dossiers
0
FDA Orange Book
0
Europe
0
Canada
0
Australia
0
South Africa
0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
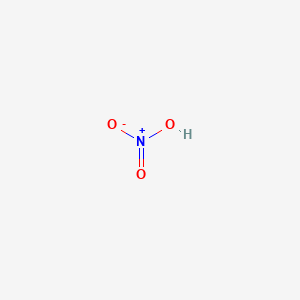

1. Acid, Nitric
1. 7697-37-2
2. Azotic Acid
3. Hydrogen Nitrate
4. Salpetersaeure
5. Aqua Fortis
6. Nitricum Acidum
7. Acide Nitrique
8. Hno3
9. Medullipin Ii
10. Nitric Acid [nf]
11. Nitric Acid, Rare Earth Salts
12. Hono2
13. Mfcd00011349
14. Chembl1352
15. 411vrn1tv4
16. Chebi:48107
17. Nitric Acid (nf)
18. 12033-49-7
19. Acidum Nitricum
20. Nitrous Fumes
21. Engraver's Acid
22. Nitryl Hydroxide
23. Red Fuming Nitric Acid
24. 122392-03-4
25. 68412-17-9
26. Rfna
27. Azotowy Kwas [polish]
28. Nitric Acid, Acs Reagent, 70%
29. Salpetersaure
30. Salpetersaure [german]
31. Zinc, Reference Standard Solution
32. Acido Nitrico
33. Cobalt, Reference Standard Solution
34. Acide Nitrique [french]
35. Acido Nitrico [italian]
36. Acido Nitrico [spanish]
37. Azotowy Kwas
38. Kyselina Dusicne
39. Kyselina Dusicne [czech]
40. Nitric Acid, Acs Reagent, >=90.0%
41. Rare Earth Nitrate
42. Nitric Acid Solution
43. Salpeterzuuroplossingen [dutch]
44. Salpeterzuuroplossingen
45. Hsdb 1665
46. Nitric Acid, Anhydrous
47. Einecs 231-714-2
48. Nitric Acid (conc 80% Or Greater)
49. Nitric Acid (red Fuming)
50. Un2031
51. Un2032
52. Nitroalcohol
53. Unii-411vrn1tv4
54. Acide Azotique
55. Nitro-alcohol
56. Nitric Acid (70% Or Less)
57. Nitro Alcohol
58. Nitric -acid
59. Trioxonitric Acid
60. Nitrosonitric Acid
61. Nitric Acid (greater Than 70%)
62. No3 Anion
63. Nitric Acid, Acs
64. Einecs 270-173-7
65. Nitric Acid, 1 M
66. (-)-pareruptorin A
67. Nitric Acid, 65%
68. Hydroxidodioxidonitrogen
69. Nitric Acid (67%)
70. Nitric Acid, Red Fuming
71. Nitric Acid, 0.1 M
72. Nitric Acid Reagent Acs
73. Nitric Acid [ii]
74. Nitric Acid [mi]
75. Nitric Acid 65%
76. Ec 231-714-2
77. Hydrogen Trioxonitrate(1-)
78. Nitric Acid, 65-70%
79. Nitric Acid [hsdb]
80. Nitric Acid [mart.]
81. Nitric Acid Concentrate, 1n
82. Wln: Pr N-o3x3
83. 78989-43-2
84. Nitric Acid, P.a., 65%
85. Nitric Acid [who-dd]
86. Nitric Acid (hno3) 94.5% By Weight Or More Hno3
87. Nitric Acid: Electronic Grade
88. Nitricum Acidum [hpus]
89. Nitric Acid, 90% Acs Grade
90. Nitric Acid, Fuming, 99.5%
91. Dtxsid5029685
92. Nitric Acid, Ar, 69-72%
93. Nitric Acid, Gallium Salt ( )
94. Nitric Acid, Lr, 69-72%
95. [no2(oh)]
96. Nitric Acid [ep Monograph]
97. Nitric Acid, Puriss., 64-66%
98. Nitric Acid, Reagent Grade, >90%
99. Bdbm50152970
100. Akos015833449
101. Nitric Acid, Technical Grade, 50.0%
102. Nsc-177677
103. Nitric Acid Other Than Red Fuming With >70% Nitric Acid [un2031] [corrosive]
104. Nitric Acid Solution, 0.1n (n/10)
105. 309963-65-3
106. Nitric Acid, 0.1n Standardized Solution
107. Nitric Acid, 1.0n Standardized Solution
108. Nitric Acid, 2.0n Standardized Solution
109. Nitric Acid, Extra Pure, 69.0-71.0%
110. Nitric Acid, Red, Fuming, Hno3 >90 %
111. Nitric Acid, Reagent Grade, Fuming, >90%
112. Nitrogen Hydroxideoxide (n(oh)2o) (9ci)
113. Ft-0689223
114. N0806
115. N1916
116. Nitric Acid, Puriss. P.a., >=65% (t)
117. C00244
118. D02313
119. Q83320
120. Nitric Acid Solution, ~32.5%, Special Quality
121. Nitric Acid, Environmental Grade Plus, 68-70%
122. Nitric Acid, 70%, Purified By Double-distillation
123. Nitric Acid, P.a., Acs Reagent, 68.0-70.0%
124. Nitric Acid, Red Fuming [un2032] [corrosive]
125. Q27110015
126. Nitric Acid Other Than Red Fuming With >70% Nitric Acid
127. Nitric Acid, Saj First Grade, 69-70%, Density: 1.42
128. 3088a493-74f8-4d19-8abc-e572420b5f29
129. Nitric Acid Other Than Red Fuming With Not >70% Nitric Acid
130. Nitric Acid, Jis Special Grade, 60.0-61.0%, Density: 1.38
131. Nitric Acid, Jis Special Grade, 65.0-66.0%, Density: 1.40
132. Nitric Acid, Jis Special Grade, 69.0-70.0%, Density: 1.42
133. Nitric Acid, Puriss. P.a., Acs Reagent, Fuming, >=99.5%
134. Nitric Acid, Puriss. P.a., Acs Reagent, Reag. Iso, >=69%
135. Nitric Acid, Saj First Grade, 60.0-62.0%, Density: 1.38
136. Nitric Acid, Saj First Grade, 65.0-66.0%, Density: 1.40
137. Nitric Acid, 68-70%, Certified Acs Plus Grade Trace Metal Analyzed
138. Nitric Acid, Jis Special Grade, >=97.0%, Fuming, Density: 1.52
139. Nitric Acid, Saj First Grade, >=97.0%, Fuming, Density: 1.52
140. Nitric Acid, 60.0-62.0%, Saj Super Special Grade, Density: 1.38
141. Nitric Acid, Jis Special Grade, 90.0-94.0%, Fuming, Density: 1.50
142. Nitric Acid, Puriss. P.a., 65% (hg <=0.0000005%), >=65% (t)
143. Nitric Acid, Saj First Grade, 90.0-94.0%, Fuming, Density: 1.50
144. Nitric Acid, Semiconductor Grade Puranal(tm) (honeywell 17818), >=65%
145. Nitric Acid, Semiconductor Grade Puranal(tm) (honeywell 17952), >=69%
146. Nitric Acid Concentrate, 0.1 M Hno3 In Water (0.1n), Eluent Concentrate For Ic
147. Nitric Acid Other Than Red Fuming With Not >70% Nitric Acid [un2031] [corrosive]
148. Nitric Acid, 60.0-62.0%, Suitable For Determination Of Toxic Metals, Density: 1.38
149. Nitric Acid, 70%, Purified By Redistillation, >=99.999% Trace Metals Basis
150. Nitric Acid, P.a., Acs Reagent, Reag. Iso, Reag. Ph. Eur., 68.5-69.5%
151. Nitric Acid, Semiconductor Grade Mos Puranal(tm) (honeywell 17920), >=69%
152. Nitric Acid, Semiconductor Grade Vlsi Puranal(tm) (honeywell 17512), 69-71%
153. Nitric Acid, 70%, Meets Analytical Specification Of Bp, Ph??eur, Ph??fran??, Usp, Fcc, E339
154. Nitric Acid, Puriss. P.a., Reag. Iso, Reag. Ph. Eur., For Determinations With Dithizone, >=65%
155. Nitric Acid, Purum P.a., Fuming, Packed In Coated, Shock- And Leak-protected Glass Bottle, >=99% (t)
| Molecular Weight | 63.013 g/mol |
|---|---|
| Molecular Formula | HNO3 |
| XLogP3 | 0.2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 0 |
| Exact Mass | 62.995642894 g/mol |
| Monoisotopic Mass | 62.995642894 g/mol |
| Topological Polar Surface Area | 66 Ų |
| Heavy Atom Count | 4 |
| Formal Charge | 0 |
| Complexity | 24.8 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
To be used for acute self-limiting conditions according to standard homeopathic indications /for warts/.
US Natl Inst Health; DailyMed. Current Medication Information for Nitric acid pellet, (October 2010). Available from, as of August 2, 2011: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=d1d02ada-5f03-4c5e-80af-13540896d355
In a double blind study, the response of superficial skin tumors to topical treatment with a nitric acid preparation (6.2 N) or Solcoderm solution was compared in 33 patients. Treatment with the Solcoderm solution yielded superior results and less reaction in the surrounding normal skin than the plain nitric acid. It was suggested that the nitrate reduction products generated in the Solcoderm preparation contribute to the improved clinical effects by enhancing the speed and intensity of tissue fixation, but not tissue erosion.
PMID:6848302 Weiner M et al; Clin Pharmacol Ther 33: 77-83 (1983)
(VET): Cauterizing agent (for warts).
Budavari, S. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. Whitehouse Station, NJ: Merck and Co., Inc., 1996., p. 1130
Explosive Agents
Substances that are energetically unstable and can produce a sudden expansion of the material, called an explosion, which is accompanied by heat, pressure and noise. Other things which have been described as explosive that are not included here are explosive action of laser heating, human performance, sudden epidemiological outbreaks, or fast cell growth. (See all compounds classified as Explosive Agents.)
The disposition of HNO3 is not easily determined. It reacts immediately with respiratory mucous membranes after inhalation and does not appear to be absorbed after oral administration.
Subcommittee on Rocket-Emission Toxicants, National Research Council; p. 197-208 in Assessment of Exposure-Response Functions for Rocket-Emission Toxicants; The National Academies Press (1998) https://www.nap.edu/
Following inhalation exposure, some HNO3 might decompose to other nitrogen oxides, which might be absorbed by the bloodstream.
Subcommittee on Rocket-Emission Toxicants, National Research Council; p. 197-208 in Assessment of Exposure-Response Functions for Rocket-Emission Toxicants; The National Academies Press (1998) https://www.nap.edu/
No reports found; [TDR, p. 937]
TDR - Ryan RP, Terry CE, Leffingwell SS (eds). Toxicology Desk Reference: The Toxic Exposure and Medical Monitoring Index, 5th Ed. Washington DC: Taylor & Francis, 1999., p. 937

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Market Place

ABOUT THIS PAGE
88
PharmaCompass offers a list of Nitric Acid API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Nitric Acid manufacturer or Nitric Acid supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Nitric Acid manufacturer or Nitric Acid supplier.
PharmaCompass also assists you with knowing the Nitric Acid API Price utilized in the formulation of products. Nitric Acid API Price is not always fixed or binding as the Nitric Acid Price is obtained through a variety of data sources. The Nitric Acid Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Nitric Acid manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Nitric Acid, including repackagers and relabelers. The FDA regulates Nitric Acid manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Nitric Acid API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Nitric Acid manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Nitric Acid supplier is an individual or a company that provides Nitric Acid active pharmaceutical ingredient (API) or Nitric Acid finished formulations upon request. The Nitric Acid suppliers may include Nitric Acid API manufacturers, exporters, distributors and traders.
click here to find a list of Nitric Acid suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Nitric Acid Drug Master File in Japan (Nitric Acid JDMF) empowers Nitric Acid API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Nitric Acid JDMF during the approval evaluation for pharmaceutical products. At the time of Nitric Acid JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Nitric Acid suppliers with JDMF on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Nitric Acid as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Nitric Acid API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Nitric Acid as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Nitric Acid and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Nitric Acid NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Nitric Acid suppliers with NDC on PharmaCompass.
Nitric Acid Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Nitric Acid GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Nitric Acid GMP manufacturer or Nitric Acid GMP API supplier for your needs.
A Nitric Acid CoA (Certificate of Analysis) is a formal document that attests to Nitric Acid's compliance with Nitric Acid specifications and serves as a tool for batch-level quality control.
Nitric Acid CoA mostly includes findings from lab analyses of a specific batch. For each Nitric Acid CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Nitric Acid may be tested according to a variety of international standards, such as European Pharmacopoeia (Nitric Acid EP), Nitric Acid JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Nitric Acid USP).

