
Synopsis
Synopsis
0
NDC API
0
VMF
0
FDA Orange Book
0
Canada
0
South Africa
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
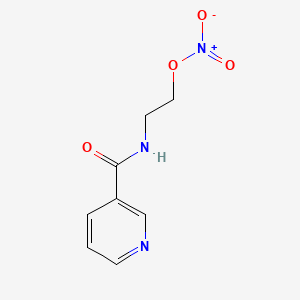

1. 2 Nicotinamidethyl Nitrate
2. 2 Nicotinamidoethyl Nitrate
3. 2-nicotinamidethyl Nitrate
4. 2-nicotinamidoethyl Nitrate
5. Adancor
6. Dancor
7. Ikorel
8. Nitrate, 2-nicotinamidethyl
9. Nitrate, 2-nicotinamidoethyl
10. Sg 75
11. Sg-75
12. Sg75
1. 65141-46-0
2. Ikorel
3. 2-nicotinamidoethyl Nitrate
4. Sigmart
5. Sg-75
6. Dancor
7. Nicorandilum
8. Adancor
9. 2-(pyridine-3-carbonylamino)ethyl Nitrate
10. 2-(nicotinamido)ethyl Nitrate
11. Sg 75
12. N-(2-hydroxyethyl)nicotinamide Nitrate
13. N-(2-hydroxyethyl)nicotinamide Nitrate (ester)
14. Nicorandil (ikorel)
15. Chebi:31905
16. N-[2-(nitrooxy)ethyl]-3-pyridinecarboxamide
17. 3-pyridinecarboxamide, N-(2-(nitroxy)ethyl)-
18. 3-pyridinecarboxamide, N-[2-(nitrooxy)ethyl]-
19. 260456ham0
20. Ncgc00025357-01
21. 2-(pyridin-3-ylformamido)ethyl Nitrate
22. 3-pyridinecarboxamide, N-(2-(nitrooxy)ethyl)-
23. Dsstox_cid_25692
24. Dsstox_rid_81064
25. Dsstox_gsid_45692
26. Nicorandilum [inn-latin]
27. 2-[(pyridin-3-ylcarbonyl)amino]ethyl Nitrate
28. 2-(nicotinamido)ethyl Nitrat
29. Smr000466365
30. Sigmart (tn)
31. Cas-65141-46-0
32. Perisalol
33. N-(2-(nitrooxy)ethyl)-3-pyridinecarboxamide
34. Sr-01000597534
35. Einecs 265-514-1
36. Brn 0481451
37. Nicorandilo
38. Unii-260456ham0
39. Nicorandil [usan:inn:ban:jan]
40. Nicorandil- Bio-x
41. Mfcd00186520
42. 2-(pyridine-3-carboxamido)ethyl Nitrate
43. Cpd000466365
44. Rp-46417
45. Nicorandil [mi]
46. Nicorandil [inn]
47. Nicorandil [jan]
48. Nicorandil [usan]
49. N-[2-(nitrooxy)ethyl]pyridine-3-carboxamide
50. Nicorandil [mart.]
51. Nicorandil [who-dd]
52. Schembl34547
53. Mls000759488
54. Mls001424162
55. Mls002222323
56. Mls006010701
57. Chembl284906
58. Gtpl2411
59. Dtxsid8045692
60. Nicorandil (jp17/usan/inn)
61. Nicorandil [ep Monograph]
62. Hms2051n16
63. Hms2089l12
64. Hms2095e14
65. Hms2232a06
66. Hms3268l19
67. Hms3371n18
68. Hms3393n16
69. Hms3413g17
70. Hms3655p05
71. Hms3677g17
72. Hms3712e14
73. Bcp09434
74. Hy-b0341
75. Zinc1533102
76. Tox21_110968
77. Bdbm50247908
78. S1971
79. Stl445540
80. Akos001589705
81. Akos015855406
82. Akos025149378
83. Tox21_110968_1
84. Ab04467
85. Ac-4690
86. Ccg-101045
87. Db09220
88. Hs-0049
89. Nc00295
90. Ncgc00025357-02
91. Ncgc00025357-04
92. 2-(pyridine-3-carbylamino) Ethyl Nitrate
93. Bn164649
94. Ft-0602677
95. N0837
96. Sw197675-3
97. D01810
98. T72780
99. Ab00639978-08
100. Ab00639978-09
101. Ab00639978_10
102. Ab00639978_11
103. A834984
104. Q862989
105. Sr-01000597534-1
106. Sr-01000597534-5
107. Brd-k97752965-001-01-6
108. F0005-2298
| Molecular Weight | 211.17 g/mol |
|---|---|
| Molecular Formula | C8H9N3O4 |
| XLogP3 | 0.8 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 4 |
| Exact Mass | 211.05930578 g/mol |
| Monoisotopic Mass | 211.05930578 g/mol |
| Topological Polar Surface Area | 97 Ų |
| Heavy Atom Count | 15 |
| Formal Charge | 0 |
| Complexity | 228 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Indicated for the prevention and treatment of chronic stable angina pectoris and reduction in the risk of acute coronary syndromes.
Nicorandil is a potassium channel opener with nitrovasodilator (NO donor) actions, making it both an arterial and a venous dilator. It causes sustained dilation of both the arterial resistance and conductive vessels that increases coronary blood flow, however the effect of the drug on coronary arteries does not involve the coronary steal phenomenon. Activation of potassium channels lead to hyperpolarization of the smooth muscle cells, followed by arterial dilation and afterload reduction. Nicorandil is shown to increase pooling in the capacitance vessels with a decrease in preload through relaxing the venous vascular system. Overall, improved blood flow and reduced infarct size are achieved through reduction of end-diastolix pressure and decreased extravascular component of vascular resistance. Open studies showed the effectiveness of nicorandil treatment on various types of angina pectoris.
Antihypertensive Agents
Drugs used in the treatment of acute or chronic vascular HYPERTENSION regardless of pharmacological mechanism. Among the antihypertensive agents are DIURETICS; (especially DIURETICS, THIAZIDE); ADRENERGIC BETA-ANTAGONISTS; ADRENERGIC ALPHA-ANTAGONISTS; ANGIOTENSIN-CONVERTING ENZYME INHIBITORS; CALCIUM CHANNEL BLOCKERS; GANGLIONIC BLOCKERS; and VASODILATOR AGENTS. (See all compounds classified as Antihypertensive Agents.)
Vasodilator Agents
Drugs used to cause dilation of the blood vessels. (See all compounds classified as Vasodilator Agents.)
Vitamin B Complex
A group of water-soluble vitamins, some of which are COENZYMES. (See all compounds classified as Vitamin B Complex.)
Anti-Arrhythmia Agents
Agents used for the treatment or prevention of cardiac arrhythmias. They may affect the polarization-repolarization phase of the action potential, its excitability or refractoriness, or impulse conduction or membrane responsiveness within cardiac fibers. Anti-arrhythmia agents are often classed into four main groups according to their mechanism of action: sodium channel blockade, beta-adrenergic blockade, repolarization prolongation, or calcium channel blockade. (See all compounds classified as Anti-Arrhythmia Agents.)
C - Cardiovascular system
C01 - Cardiac therapy
C01D - Vasodilators used in cardiac diseases
C01DX - Other vasodilators used in cardiac diseases
C01DX16 - Nicorandil
Absorption
Following oral administration, nicorandil is well absorbed from the gastrointestinal tract with the oral bioavailability of 75% with the maximum peak plasma concentration (Cmax) reached within 30-60 minutes. The mean Cmax is Cmax then is approximately 300 ng/ml. Steady-state plasma concentrations of nicorandil usually are reached within approximately 96-120 h after twice daily dosing (10 or 20mg).
Route of Elimination
The main route of elimination is the kidney with more than 60% of the administered dose was eliminated in the urine 24 hours after dosing. Only approximately 1% of nicorandil is excreted unchanged in the urine, and the remaining compounds are mainly the denitrated metabolite (9%) and its derivatives (e.g. nicotinuric acid 6%, nicotinamide 1%, N-methylnicotinamide < 1% and nicotinic acid < 1%). Less than 2% of administered dose is excreted through the biliary system.
Volume of Distribution
After oral (and i.v.) administration of the drug, the apparent volume of distribution is approximately 1.0-1.4 L/kg body weight.
Clearance
The total body clearance is approximately 1.15 L/min.
Nicorandil undergoes extensive hepatic metabolism. The main biotransformation pathways of nicorandil are denitration, followed by subsequent nicotinamide metabolism. The main pharmacologically inactive denitrated metabolite 2-nicotinamidoethanol can be detected in the urine. The derivatives formed from the nicotinamide metabolism of denitrated products are nicotinuric acid, nicotinamide, N-methylnicotinamide and nicotinic acid.
The elimination half life is approximately 1 hour.
Nicorandil mediates its therapeutic efficacy via two main mechanisms. Nicorandil is an activator and opener of ATP-sensitive (ATP-dependent) potassium channels (KATP channels) that are composed of Kir6.x-type subunits and sulfonylurea receptor (SUR) subunits. Nicorandil binding sites are located in the sulfonylurea receptor 2 (SUR2) in the ATP-sensitive potassium channel, which are regulatory subunits of the channel that exhibit an ATPase activitiy. There are 2 types of SUR2 subunits (2A/2B) that have identical nucleotide binding domains (NBD), where SUR2A is more predominantly expressed in skeletal and cardiac myocytes and SUR2B in smooth muscle cells. Nicorandil more potently activates SUR2B/Kir6.2 than SUR2A/Kir6.2 channels to cause hyperpolarization. ATP-NBD1 interaction influences the channel signalling by nicorandil, and the response of the channel to nicorandil is also facilitated and heightened by the interaction of ATP or ADP with NBD2. Potentiated activity of ATP-sensitive channels have cardioprotective role by limiting the duration of action potentials and preventing intraceullar calcium overload. This attenuates cellular injury by preserving cellular energetics and ultimately cell survival. KATP channel-dependent membrane hyperpolarization can also lead to vasodilation via reduction in Ca2+ influx through the voltage-gated Ca2+ channels and regulation of intracellular Ca2+ mobilization in smooth muscle cells. Nicorandil contain a nitrate moiety in its structure, making it a good dilator of vascular smooth muscle like other nitroglycerin esters. Direct relaxation of venous vascular system arises from NO-donor mediated stimulation of guanylyl cyclase and increased levels of intracellular cyclic GMP (cGMP). Elevated levels of cGMP contributes to the total relaxing effect of nicorandil at higher concentrations of the drug.
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : CEP 2023-426 - Rev 00
Status : Valid
Issue Date : 2024-08-09
Type : Chemical
Substance Number : 2332

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] 
API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
92
PharmaCompass offers a list of Nicorandil API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Nicorandil manufacturer or Nicorandil supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Nicorandil manufacturer or Nicorandil supplier.
PharmaCompass also assists you with knowing the Nicorandil API Price utilized in the formulation of products. Nicorandil API Price is not always fixed or binding as the Nicorandil Price is obtained through a variety of data sources. The Nicorandil Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Nicorandil manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Nicorandil, including repackagers and relabelers. The FDA regulates Nicorandil manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Nicorandil API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Nicorandil manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Nicorandil supplier is an individual or a company that provides Nicorandil active pharmaceutical ingredient (API) or Nicorandil finished formulations upon request. The Nicorandil suppliers may include Nicorandil API manufacturers, exporters, distributors and traders.
click here to find a list of Nicorandil suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Nicorandil DMF (Drug Master File) is a document detailing the whole manufacturing process of Nicorandil active pharmaceutical ingredient (API) in detail. Different forms of Nicorandil DMFs exist exist since differing nations have different regulations, such as Nicorandil USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Nicorandil DMF submitted to regulatory agencies in the US is known as a USDMF. Nicorandil USDMF includes data on Nicorandil's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Nicorandil USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Nicorandil suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Nicorandil Drug Master File in Japan (Nicorandil JDMF) empowers Nicorandil API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Nicorandil JDMF during the approval evaluation for pharmaceutical products. At the time of Nicorandil JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Nicorandil suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Nicorandil Drug Master File in Korea (Nicorandil KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Nicorandil. The MFDS reviews the Nicorandil KDMF as part of the drug registration process and uses the information provided in the Nicorandil KDMF to evaluate the safety and efficacy of the drug.
After submitting a Nicorandil KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Nicorandil API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Nicorandil suppliers with KDMF on PharmaCompass.
A Nicorandil CEP of the European Pharmacopoeia monograph is often referred to as a Nicorandil Certificate of Suitability (COS). The purpose of a Nicorandil CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Nicorandil EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Nicorandil to their clients by showing that a Nicorandil CEP has been issued for it. The manufacturer submits a Nicorandil CEP (COS) as part of the market authorization procedure, and it takes on the role of a Nicorandil CEP holder for the record. Additionally, the data presented in the Nicorandil CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Nicorandil DMF.
A Nicorandil CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Nicorandil CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Nicorandil suppliers with CEP (COS) on PharmaCompass.
A Nicorandil written confirmation (Nicorandil WC) is an official document issued by a regulatory agency to a Nicorandil manufacturer, verifying that the manufacturing facility of a Nicorandil active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Nicorandil APIs or Nicorandil finished pharmaceutical products to another nation, regulatory agencies frequently require a Nicorandil WC (written confirmation) as part of the regulatory process.
click here to find a list of Nicorandil suppliers with Written Confirmation (WC) on PharmaCompass.
Nicorandil Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Nicorandil GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Nicorandil GMP manufacturer or Nicorandil GMP API supplier for your needs.
A Nicorandil CoA (Certificate of Analysis) is a formal document that attests to Nicorandil's compliance with Nicorandil specifications and serves as a tool for batch-level quality control.
Nicorandil CoA mostly includes findings from lab analyses of a specific batch. For each Nicorandil CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Nicorandil may be tested according to a variety of international standards, such as European Pharmacopoeia (Nicorandil EP), Nicorandil JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Nicorandil USP).

