


API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
0
Listed Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
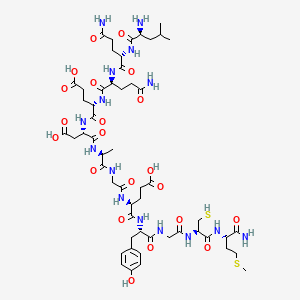

1. Lissamine-rhodamine Dodecanoic Acid
2. Lr-12
3. Motrem
1. Dtxsid201336482
2. 2014384-91-7
| Molecular Weight | 1342.5 g/mol |
|---|---|
| Molecular Formula | C54H83N15O21S2 |
| XLogP3 | -8.3 |
| Hydrogen Bond Donor Count | 20 |
| Hydrogen Bond Acceptor Count | 24 |
| Rotatable Bond Count | 45 |
| Exact Mass | g/mol |
| Monoisotopic Mass | g/mol |
| Topological Polar Surface Area | 634 |
| Heavy Atom Count | 92 |
| Formal Charge | 0 |
| Complexity | 2630 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 10 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anti-Inflammatory Agents
Substances that reduce or suppress INFLAMMATION. (See all compounds classified as Anti-Inflammatory Agents.)
Nangibotide targets the immunoreceptor TREM-1 (triggering receptor expressed on myeloid cells-1), and has been explored in treating inflammatory disorders like septic schock. Safety and pharmacokinetics studies have shown Nangibotide to be safe and well-tolerated in humans, while animal septic shock models have shown its ability to restore vascular function and improve survival. As of July 2020, Inotrem is recuriting patients to study Nangibotide's effects on patients with COVID-19 and systemic inflammation (NCT04429334).


