
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
FDF Dossiers
0
FDA Orange Book
0
Europe
0
Canada
0
Australia
0
South Africa
0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
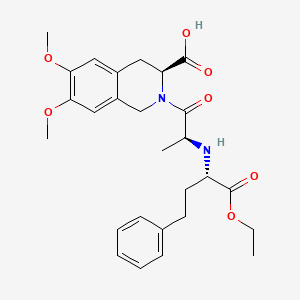

1. 2-((1-ethoxycarbony)-3-phenylpropylamino-1-oxopropyl)-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline-3-carboxylic Acid
2. Fempress
3. Moex
4. Moexipril Hydrochloride
5. Perdix
6. Rs 10085
7. Rs-10085
8. Univasc
1. 103775-10-6
2. Uniretic
3. Univasc
4. Moexiprilum [inn-latin]
5. (3s)-2-[(2s)-2-[[(2s)-1-ethoxy-1-oxo-4-phenylbutan-2-yl]amino]propanoyl]-6,7-dimethoxy-3,4-dihydro-1h-isoquinoline-3-carboxylic Acid
6. Moexipril (inn)
7. Ci-925
8. 109715-88-0
9. Rs-10085
10. Chembl1165
11. Wt87c52tjz
12. Chebi:6960
13. Moexiprilum
14. Moexipril [inn]
15. Moexipril [inn:ban]
16. (3s)-2-((2s)-n-((1s)-1-carboxy-3-phenylpropyl)alanyl)-1,2,3,4-tetrahydro-6,7-dimethoxy-3-isoquinolinecarboxylic Acid, 2-ethyl Ester
17. (s)-2-(((s)-1-ethoxy-1-oxo-4-phenylbutan-2-yl)-l-alanyl)-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline-3-carboxylic Acid
18. (s)-2-((s)-2-(((s)-1-ethoxy-1-oxo-4-phenylbutan-2-yl)amino)propanoyl)-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline-3-carboxylic Acid
19. (s)-2-((s)-2-((s)-1-ethoxy-1-oxo-4-phenylbutan-2-ylamino)propanoyl)-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline-3-carboxylic Acid
20. 3-isoquinolinecarboxylic Acid,2-[(2s)-2-[[(1s)-1-(ethoxycarbonyl)-3-phenylpropyl]amino]-1-oxopropyl]-1,2,3,4-tetrahydro-6,7-dimethoxy-, (3s)-
21. Unii-wt87c52tjz
22. Moexipril [mi]
23. Moexipril [vandf]
24. Moexipril [who-dd]
25. Schembl34030
26. Bidd:gt0007
27. Gtpl6571
28. Dtxsid9023330
29. Zinc3812306
30. Bdbm50084673
31. Akos015843317
32. Db00691
33. Ncgc00263546-03
34. (3s)-2-(n-{(1s)-1-[(ethyloxy)carbonyl]-3-phenylpropyl}-l-alanyl)-6,7-bis(methyloxy)-1,2,3,4-tetrahydroisoquinoline-3-carboxylic Acid
35. Hy-117281
36. Cs-0064930
37. 75m106
38. C07704
39. D08225
40. Ab01565830_02
41. A800803
42. Q2291605
43. Brd-k34441861-003-01-3
44. 4-(4-oxopiperidine-1-carbonyl)phenylboronicacid
45. (3s)-2-((2s)-2-(((1s)-1-(ethoxycarbonyl)-3-phenylpropyl)amino)-1-oxopropyl)-1,2,3,4-tetrahydro-6,7-dimethoxy-3-isoquinolinecarboxylic Acid
46. (3s)-2-[(2s)-2-{[(2s)-1-ethoxy-1-oxo-4-phenylbutan-2-yl]amino}propanoyl]-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline-3-carboxylic Acid
47. 1-[2-(1-ethoxycarbonyl-3-phenyl-propylamino)-propionyl]-octahydro-indole-2-carboxylic Acid(moexipril)
48. 3-isoquinolinecarboxylic Acid, 2-(2-((1-(ethoxycarbonyl)-3-phenylpropyl)amino)-1-oxopropyl)-1,2,3,4-tetrahydro-6,7-dimethoxy-, (3s-(2(r*(r*)),3r*))-
| Molecular Weight | 498.6 g/mol |
|---|---|
| Molecular Formula | C27H34N2O7 |
| XLogP3 | 1.2 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 12 |
| Exact Mass | 498.23660143 g/mol |
| Monoisotopic Mass | 498.23660143 g/mol |
| Topological Polar Surface Area | 114 Ų |
| Heavy Atom Count | 36 |
| Formal Charge | 0 |
| Complexity | 742 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For the treatment of hypertension.
Moexipril is a non-sulfhydryl containing precursor of the active angiotensin-converting enzyme (ACE) inhibitor moexiprilat. It is used to treat high blood pressure (hypertension). It works by relaxing blood vessels, causing them to widen. Lowering high blood pressure helps prevent strokes, heart attacks and kidney problems.
Angiotensin-Converting Enzyme Inhibitors
A class of drugs whose main indications are the treatment of hypertension and heart failure. They exert their hemodynamic effect mainly by inhibiting the renin-angiotensin system. They also modulate sympathetic nervous system activity and increase prostaglandin synthesis. They cause mainly vasodilation and mild natriuresis without affecting heart rate and contractility. (See all compounds classified as Angiotensin-Converting Enzyme Inhibitors.)
Antihypertensive Agents
Drugs used in the treatment of acute or chronic vascular HYPERTENSION regardless of pharmacological mechanism. Among the antihypertensive agents are DIURETICS; (especially DIURETICS, THIAZIDE); ADRENERGIC BETA-ANTAGONISTS; ADRENERGIC ALPHA-ANTAGONISTS; ANGIOTENSIN-CONVERTING ENZYME INHIBITORS; CALCIUM CHANNEL BLOCKERS; GANGLIONIC BLOCKERS; and VASODILATOR AGENTS. (See all compounds classified as Antihypertensive Agents.)
C - Cardiovascular system
C09 - Agents acting on the renin-angiotensin system
C09A - Ace inhibitors, plain
C09AA - Ace inhibitors, plain
C09AA13 - Moexipril
Absorption
Moexipril is incompletely absorbed, with bioavailability as moexiprilat of about 13% compared to intravenous (I.V.) moexipril (both measuring the metabolite moexiprilat), and is markedly affected by food, which reduces Cmax and AUC by about 70% and 40%, respectively, after the ingestion of a low-fat breakfast or by 80% and 50%, respectively, after the ingestion of a high-fat breakfast.
Route of Elimination
Moexiprilat undergoes renal elimination.
Volume of Distribution
183 L
Clearance
441 mL/min
Rapidly converted to moexiprilat, the active metabolite. Conversion to the active metabolite is thought to require carboxyesterases and is likely to occur in organs or tissues, other than the gastrointestinal tract, in which carboxyesterases occur. The liver is thought to be one site of conversion, but not the primary site.
Moexipril elimination half-life is approximately 1 hour. Moexiprilat elimination half-life is 2 to 9 hours.
Moexipril is a prodrug for moexiprilat, which inhibits ACE in humans and animals. The mechanism through which moexiprilat lowers blood pressure is believed to be primarily inhibition of ACE activity. ACE is a peptidyl dipeptidase that catalyzes the conversion of the inactive decapeptide angiotensin I to the vasoconstrictor substance angiotensin II. Angiotensin II is a potent peripheral vasoconstrictor that also stimulates aldosterone secretion by the adrenal cortex and provides negative feedback on renin secretion. ACE is identical to kininase II, an enzyme that degrades bradykinin, an endothelium-dependent vasodilator. Moexiprilat is about 1000 times as potent as moexipril in inhibiting ACE and kininase II. Inhibition of ACE results in decreased angiotensin II formation, leading to decreased vasoconstriction, increased plasma renin activity, and decreased aldosterone secretion. The latter results in diuresis and natriuresis and a small increase in serum potassium concentration (mean increases of about 0.25 mEq/L were seen when moexipril was used alone). Whether increased levels of bradykinin, a potent vasodepressor peptide, play a role in the therapeutic effects of moexipril remains to be elucidated. Although the principal mechanism of moexipril in blood pressure reduction is believed to be through the renin-angiotensin-aldosterone system, ACE inhibitors have some effect on blood pressure even in apparent low-renin hypertension.
ABOUT THIS PAGE
57
PharmaCompass offers a list of Moexiprilum API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Moexiprilum manufacturer or Moexiprilum supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Moexiprilum manufacturer or Moexiprilum supplier.
PharmaCompass also assists you with knowing the Moexiprilum API Price utilized in the formulation of products. Moexiprilum API Price is not always fixed or binding as the Moexiprilum Price is obtained through a variety of data sources. The Moexiprilum Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Moexiprilum manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Moexiprilum, including repackagers and relabelers. The FDA regulates Moexiprilum manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Moexiprilum API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Moexiprilum manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Moexiprilum supplier is an individual or a company that provides Moexiprilum active pharmaceutical ingredient (API) or Moexiprilum finished formulations upon request. The Moexiprilum suppliers may include Moexiprilum API manufacturers, exporters, distributors and traders.
click here to find a list of Moexiprilum suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
Moexiprilum Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Moexiprilum GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Moexiprilum GMP manufacturer or Moexiprilum GMP API supplier for your needs.
A Moexiprilum CoA (Certificate of Analysis) is a formal document that attests to Moexiprilum's compliance with Moexiprilum specifications and serves as a tool for batch-level quality control.
Moexiprilum CoA mostly includes findings from lab analyses of a specific batch. For each Moexiprilum CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Moexiprilum may be tested according to a variety of international standards, such as European Pharmacopoeia (Moexiprilum EP), Moexiprilum JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Moexiprilum USP).

