Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
FDF Dossiers
0
FDA Orange Book
0
Europe
0
Canada
0
Australia
0
South Africa
0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
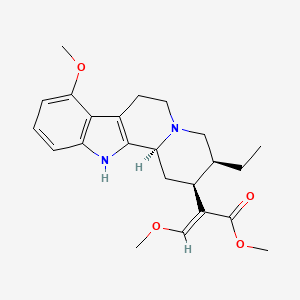

1. Mitragynine
2. 16,17-didehydro-9,17-dimethoxy-17,18-seco-20-alpha-yohimban-16-carboxylic Acid Methyl Ester
1. Mitragynine
2. 4098-40-2
3. 9-methoxycorynantheidine
4. Skf 12711
5. Ep479k822j
6. Dtxsid701032140
7. Methyl (e)-2-[(2s,3s,12bs)-3-ethyl-8-methoxy-1,2,3,4,6,7,12,12b-octahydroindolo[2,3-a]quinolizin-2-yl]-3-methoxyprop-2-enoate
8. 16,17-didehydro-9,17-dimethoxy-17,18-seco-20-alpha-yohimban-16-carboxylic Acid Methyl Ester
9. Methyl (2e)-2-((2s,4s,5s)-5-ethyl-12-methoxy-7,17-diazatetracyclo(8.7.0.0^(2,7).0^(11,16))heptadeca-1(10),11,13,15-tetraen-4-yl)-3-methoxyprop-2-enoate
10. Methyl (2e)-2-[(2s,4s,5s)-5-ethyl-12-methoxy-7,17-diazatetracyclo[8.7.0.0^{2,7}.0^{11,16}]heptadeca-1(10),11,13,15-tetraen-4-yl]-3-methoxyprop-2-enoate
11. Methyl (e)-2-((2s,3s,12bs)-3-ethyl-8-methoxy-1,2,3,4,6,7,12,12b-octahydroindolo(2,3-a)quinolizin-2-yl)-3-methoxyprop-2-enoate
12. Refchem:159115
13. Dtxcid701517205
14. Sk&f-12711
15. Chebi:6956
16. Chembl299031
17. Mitragynine Picrate
18. C23h30n2o4
19. (e)-methyl 2-((2s,3s,12bs)-3-ethyl-8-methoxy-1,2,3,4,6,7,12,12b-octahydroindolo[2,3-a]quinolizin-2-yl)-3-methoxyacrylate
20. Mitragynine (100?g/ml In Methanol)
21. Mitragynin
22. Unii-ep479k822j
23. Hsdb 7901
24. Mitragynine [mi]
25. Sk&f 12711
26. Mitragynine - 95%
27. Mitragynine - 97%
28. Mitragynine [who-dd]
29. Schembl875799
30. Schembl30227792
31. Gtpl13149
32. Mitragynine, 1mg/ml In Methanol
33. Lelbftmxciikkx-qvrqzemusa-n
34. Glxc-02988
35. Mitragynine, 0.1mg/ml In Methanol
36. Msk40382
37. Bdbm50474152
38. Ex-a14763
39. Fm26024
40. Mitragynine 100 Microg/ml In Methanol
41. Ncgc00488797-01
42. Bm164595
43. Methyl (e)-2-[(2s,3s,12bs)-3-ethyl-8-methoxy-1,2,3,4,6,7,12,12b-octahydroindolo[2,3-a]quinolizin-2-yl]-3-methoxy-prop-2-enoate
44. Db-299985
45. C09226
46. Q414299
47. Corynan-16-carboxylic Acid, 16,17-didehydro-9,17-dimethoxy-, Methyl Ester, (16e,20beta)-
48. (a-e,2s,3s,12bs)-3-ethyl-1,2,3,4,6,7,12 ,12b-octahydro-8-methoxy-a-(methoxymethylene)-indolo[2 ,3-a]quinolizine-2-acetic Acid Methyl Ester
49. (e)-methyl2-((2s,3s,12bs)-3-ethyl-8-methoxy-1,2,3,4,6,7,12,12b-octahydroindolo[2,3-a]quinolizin-2-yl)-3-methoxyacrylate
50. Indolo[2,3-a]quinolizine-2-acetic Acid, 3-ethyl-1,2,3,4,6,7,12,12b-octahydro-8-methoxy-alpha-(methoxymethylene)-, Methyl Ester, (alphae,2s,3s,12bs)-
51. Methyl(e)-2-[(2s,3s,12bs)-3-ethyl-8-methoxy-1,2,3,4,6,7,12,12b-octahydroindolo[2,3-a]quinolizin-2-yl]-3-methoxyprop-2-enoate
| Molecular Weight | 398.5 g/mol |
|---|---|
| Molecular Formula | C23H30N2O4 |
| XLogP3 | 3.4 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 6 |
| Exact Mass | Da |
| Monoisotopic Mass | Da |
| Topological Polar Surface Area | 63.8 |
| Heavy Atom Count | 29 |
| Formal Charge | 0 |
| Complexity | 624 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
... LC-MS/MS analysis... was applied to quantify mitragynine in plasma samples of rats (n=8 per sampling time) treated with a single oral dose of 20 mg/kg. The following pharmacokinetic parameters were obtained (mean): maximum plasma concentration: 424 ng/mL; time to reach maximum plasma concentration: 1.26 hr; elimination half-life: 3.85 hr, apparent total clearance: 6.35 L/hr/kg, and apparent volume of distribution: 37.90 L/kg.
PMID:19589735 de Moraes NV et al; J Chromatog B Analyt Technol Biomed Life Sci 877 (24): 2593-7 (2009)
Mitragyna speciosa (Kratom) is ... a drug of abuse. When monitoring its abuse in urine, several alkaloids and their metabolites must be considered. In former studies, mitragynine (MG), its diastereomer speciogynine (SG), and paynantheine and their metabolites could be identified in rat and human urine using /Liquid Chromatography - Tandem Mass Spectometry/ (LC-MS(n)). In Kratom users' urines, besides MG and SG, further isomeric compounds were detected. To elucidate whether the MG and SG diastereomer speciociliatine (SC) and its metabolites represent further compounds, the phase I and II metabolites of SC were identified first in rat urine after the administration of the pure alkaloid. Then, the identified rat metabolites were screened for in the urine of Kratom users using the above-mentioned LC-MS(n) procedure. Considering the mass spectra and retention times, it could be confirmed that SC and its metabolites are so far the unidentified isomers in human urine. In conclusion, SC and its metabolites can be used as further markers for Kratom use, especially by consumption of raw material or products that contain a high amount of fruits of the Malaysian plant M. speciosa.
PMID:21249338 Philipp AA et al; Anal Bioanal Chem 399 (8): 2747-53 (2011)
... The aim of /this/ study is to identify the phase I and II metabolites of mitragynine (MG) in rat and human urine after solid-phase extraction (SPE) using liquid chromatography-linear ion trap mass spectrometry providing detailed structure information in the MSn mode particularly with high resolution. The seven identified phase I metabolites indicated that MG was metabolized by hydrolysis of the methylester in position 16, O-demethylation of the 9-methoxy group and of the 17-methoxy group, followed, via the intermediate aldehydes, by oxidation to carboxylic acids or reduction to alcohols and combinations of some steps. In rats, four metabolites were additionally conjugated to glucuronides and one to sulfate, but in humans, three metabolites to glucuronides and three to sulfates.
PMID:19536806 Philipp AA et al; J Mass Spectrom 44 (8): 1249-61 (2009)
During studies on the main Kratom alkaloid mitragynine (MG) in rats and humans, several dehydro analogs could be detected in urine of Kratom users, which were not found in rat urine after administration of pure MG. Questions arose as to whether these compounds are formed from MG only by humans or whether they are metabolites formed from the second abundant Kratom alkaloid paynantheine (PAY), the dehydro analog of MG. Therefore, the aim of /this/ study was to identify the phase I and II metabolites of PAY in rat urine after administration of the pure alkaloid. This was first isolated from Kratom leaves. Liquid chromatography-linear ion trap mass spectrometry provided detailed structure information of the metabolites in the MS(n) mode particularly with high resolution. Besides PAY, the following phase I metabolites could be identified: 9-O-demethyl PAY, 16-carboxy PAY, 9-O-demethyl-16-carboxy PAY, 17-O-demethyl PAY, 17-O-demethyl-16,17-dihydro PAY, 9,17-O-bisdemethyl PAY, 9,17-O-bisdemethyl-16,17-dihydro PAY, 17-carboxy-16,17-dihydro PAY, and 9-O-demethyl-17-carboxy-16,17-dihydro PAY. These metabolites indicated that PAY was metabolized via the same pathways as MG. Several metabolites were excreted as glucuronides or sulfates. The metabolism studies in rats showed that PAY and its metabolites corresponded to the MG-related dehydro compounds detected in urine of the Kratom users. In conclusion, PAY and its metabolites may be further markers for a Kratom abuse in addition of MG and its metabolites.
PMID:19902190 Philipp AA et al; Anal Bioanal Chem 396 (7): 2379-91 (2010)
... Mitragynine (MIT), a mu-opioid agonist with antinociceptive and antitussive properties...
PMID:19589735 de Moraes NV et al; J Chromatog B Analyt Technol Biomed Life Sci 877 (24): 2593-7 (2009)
Mitragynine, the major alkaloid identified from Kratom, has been reported as a partial opioid agonist producing similar effects to morphine. An interesting minor alkaloid of Kratom, 7-hydroxymitragynine, has been reported to be more potent than morphine. Both Kratom alkaloids are reported to activate supraspinal mu- and delta- opioid receptors, explaining their use by chronic narcotics users to ameliorate opioid withdrawal symptoms.
PMID:18259963 Babu KM et al; Clon Toxicol (Phila) 46 (2): 146-52 (2008)
ABOUT THIS PAGE

