
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
FDA Orange Book
0
Canada
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Amidone
2. Biodone
3. Dolophine
4. Hydrochloride, Methadone
5. Metadol
6. Metasedin
7. Methaddict
8. Methadone Hydrochloride
9. Methadose
10. Methex
11. Phenadone
12. Phymet
13. Physeptone
14. Pinadone
15. Symoron
1. Dl-methadone
2. Phenadone
3. Diaminon
4. Dolophin
5. Heptadone
6. Amidone
7. Methadon
8. Adanon
9. Physeptone
10. Ketalgin
11. 76-99-3
12. Algovetin
13. Heptanon
14. Sedo-rapide
15. Racemic Methadone
16. Metasedin
17. (+-)-methadone
18. (+/-)-methadone
19. 6-(dimethylamino)-4,4-diphenylheptan-3-one
20. Methadonum
21. 3-heptanone, 6-(dimethylamino)-4,4-diphenyl-
22. Heptanon (pharmaceutical)
23. Eptadone
24. 6-dimethylamino-4,4-diphenyl-3-heptanone
25. Althose
26. 6-(dimethylamino)-4,4-diphenyl-3-heptanone
27. Uc6vbe7v1z
28. Rac-methadone
29. Chebi:6807
30. Ids-nm-002
31. Metadone
32. (+/-)-methadone Hydrochloride
33. Metadone [dcit]
34. Metadona [spanish]
35. Methadonum [latin]
36. Methadona [spanish]
37. Metadona
38. Methadona
39. Dolophine Hcl
40. Methadone [inn:ban]
41. Metadona [inn-spanish]
42. Methadonum [inn-latin]
43. Symoron
44. Methadone (ban)
45. Hsdb 3119
46. K 174
47. 3-14-00-00279 (beilstein Handbook Reference)
48. Einecs 200-996-9
49. Unii-uc6vbe7v1z
50. A 4624
51. Fenadone (*hydrochloride*)
52. Heptanon (*hydrochloride*)
53. Brn 3213669
54. Dolophine (*hydrochloride*)
55. Heptadone (*hydrochloride*)
56. Levomethadon
57. Phenadone (*hydrochloride*)
58. An-148 (*hydrochloride*)
59. Dea No. 9250
60. (rs)-methadones
61. (plusmn)-methadone
62. Phy
63. Hoescht 10820 (*hydrochloride*)
64. Mecodin (salt/mix)
65. Methadone-hcl,(-)
66. Polamidon (salt/mix)
67. Polamidone (salt/mix)
68. Methadone [inn]
69. Methadone [mi]
70. Methadone [hsdb]
71. (.+/-.)-methadone
72. Methadone [vandf]
73. Chembl651
74. Methadone [who-dd]
75. Schembl34140
76. Divk1c_000963
77. Gtpl5458
78. Adanon Hydrochloride (salt/mix)
79. Dtxsid7023273
80. Bdbm82507
81. Kbio1_000963
82. Chebi:167309
83. Ninds_000963
84. Nsc_22266
85. Stl455106
86. Akos015962259
87. Rac-methadone 0.1 Mg/ml In Methanol
88. Rac-methadone 1.0 Mg/ml In Methanol
89. Db00333
90. Idi1_000963
91. Ncgc00248116-01
92. Ncgc00248116-02
93. Ac-16055
94. 2-dimethylamino-4,4-diphenyl-5-heptanone
95. Cas_5967-73-7
96. Db-053574
97. 6-(dimethylamino)-4,4-diphenyl-heptan-3-one
98. C07163
99. D08195
100. L000874
101. Q179996
102. 3-heptanone, 6-(dimethylamino)-4,4-diphenyl-, (.+/-.)-
103. (+/-)-methadone Solution, 1 Mg/ml In Methanol, Ampule Of 1 Ml, Certified Reference Material
104. (+/-)-methadone Solution, 100 Mug/ml In Methanol, Ampule Of 1 Ml, Certified Reference Material
| Molecular Weight | 309.4 g/mol |
|---|---|
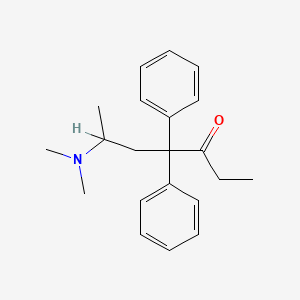
| Molecular Formula | C21H27NO |
| XLogP3 | 3.9 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 7 |
| Exact Mass | 309.209264485 g/mol |
| Monoisotopic Mass | 309.209264485 g/mol |
| Topological Polar Surface Area | 20.3 Ų |
| Heavy Atom Count | 23 |
| Formal Charge | 0 |
| Complexity | 346 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Analgesics, Opioid; Antitussive Agents; Narcotics
National Library of Medicine's Medical Subject Headings. Methadone. Online file (MeSH, 2017). Available from, as of October 2, 2017: https://meshb.nlm.nih.gov/search
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Methadone is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of October 2, 2017: https://clinicaltrials.gov/
Methadone hydrochloride tablets are indicated for the: Detoxification treatment of opioid addiction (heroin or other morphine-like drugs); Maintenance treatment of opioid addiction (heroin or other morphine-like drugs), in conjunction with appropriate social and medical services. /Included in US product label/
NIH; DailyMed. Current Medication Information for Methadone Hydrochloride Tablet (Updated: April 2017). Available from, as of October 2, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=eddf7077-02fb-4771-9823-31984f4ff2bb
Methadone hydrochloride tablets /is/ indicated for the management of pain severe enough to require daily, around-the-clock, long-term opioid treatment and for which alternative treatment options are inadequate. /Included in US product label/
NIH; DailyMed. Current Medication Information for Methadone Hydrochloride Tablet (Updated: April 2017). Available from, as of October 2, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=eddf7077-02fb-4771-9823-31984f4ff2bb
VET: Methadone may be used as an alternative opioid preanesthetic or analgesic in dogs or cats. It is also being investigated for epidural use for horses. Poor oral bioavailability precludes oral dosing in dogs.
Plumb D.C. Veterinary Drug Handbook. 8th ed. (pocket). Ames, IA: Wiley-Blackwell, 2015., p. 936
/BOXED WARNING/ ADDICTION, ABUSE, AND MISUSE: Methadone hydrochloride tablets expose patients and other users to the risks of opioid addiction, abuse, and misuse, which can lead to overdose and death. Assess each patient's risk prior to prescribing methadone hydrochloride tablets, and monitor all patients regularly for the development of these behaviors and conditions
NIH; DailyMed. Current Medication Information for Methadone Hydrochloride Tablet (Updated: April 2017). Available from, as of October 2, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=eddf7077-02fb-4771-9823-31984f4ff2bb
/BOXED WARNING/ LIFE-THREATENING RESPIRATORY DEPRESSION: Serious, life-threatening, or fatal respiratory depression may occur with use of methadone hydrochloride tablets. The peak respiratory depressant effect of methadone occurs later, and persists longer than the peak analgesic effect, especially during the initial dosing period. Monitor for respiratory depression, especially during initiation of methadone hydrochloride tablets or following a dose increase.
NIH; DailyMed. Current Medication Information for Methadone Hydrochloride Tablet (Updated: April 2017). Available from, as of October 2, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=eddf7077-02fb-4771-9823-31984f4ff2bb
/BOXED WARNING/ ACCIDENTAL INGESTION: Accidental ingestion of even one dose of methadone hydrochloride tablets, especially by children, can result in a fatal overdose of methadone.
NIH; DailyMed. Current Medication Information for Methadone Hydrochloride Tablet (Updated: April 2017). Available from, as of October 2, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=eddf7077-02fb-4771-9823-31984f4ff2bb
/BOXED WARNING/ LIFE-THREATENING QT PROLONGATION: QT interval prolongation and serious arrhythmia (torsades de pointes) have occurred during treatment with methadone. Most cases involve patients being treated for pain with large, multiple daily doses of methadone, although cases have been reported in patients receiving doses commonly used for maintenance treatment of opioid addiction. Closely monitor patients with risk factors for development of prolonged QT interval, a history of cardiac conduction abnormalities, and those taking medications affecting cardiac conduction for changes in cardiac rhythm during initiation and titration of methadone hydrochloride tablets.
NIH; DailyMed. Current Medication Information for Methadone Hydrochloride Tablet (Updated: April 2017). Available from, as of October 2, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=eddf7077-02fb-4771-9823-31984f4ff2bb
For more Drug Warnings (Complete) data for Methadone (34 total), please visit the HSDB record page.
A case of death attributed to methadone acute poisoning in an infant aged 11 months is reported. A sudden infant death syndrome (SIDS) was suspected, whereas a traumatic cause of death was excluded regarding autopsy findings. ... Methadone and its metabolite (EDDP) were detected in all the tested fluids, as well as in hair, with a blood concentration of methadone considered as lethal for children (73 ng/mL). ...
PMID:24588273 Tournel G et al; J Forensic Sci 59 (5): 1436-40 (2014)
Therapeutic methadone blood concentration: 30-100 ug/dL; Toxic methadone blood concentration: 200 ug/dL; Lethal methadone blood concentration: >400 ug/dL /From table/
Gossel, T.A., J.D. Bricker. Principles of Clinical Toxicology. 3rd ed. New York, NY: Raven Press, Ltd., 1994., p. 298
Doses of 50 mg or less have been known to be lethal to nontolerant adults. The fatal dose in children is 10-20 mg.
Ellenhorn, M.J. and D.G. Barceloux. Medical Toxicology - Diagnosis and Treatment of Human Poisoning. New York, NY: Elsevier Science Publishing Co., Inc. 1988., p. 714
A chronic pain patient prescribed 20 mg of methadone per day was seen at the Emergency Department within one hour following a witnessed intentional 200 mg ingestion. In addition, he was taking the serotonin re-uptake inhibitor antidepressant drugs, sertraline and venlafaxine as prescribed. Methadone is also a serotonin re-uptake inhibitor which has been involved in serotonin toxicity reactions. Initially, no symptoms of narcotic overdose (depressed central nervous system, respiration, or blood pressure) could be distinguished, and the standard narcotic urine screen was negative. No decontamination or antagonist therapy was given, and the patient was discharged to a psychiatric unit for observation. At 5 hours post-ingestion he presented in a panic with hallucinations and elevated blood pressure, pulse, and respiration. These symptoms are characteristic of serotonin syndrome which is often described as mental status changes, autonomic hyperactivity, and neuromuscular abnormalities. At 10 hours post-ingestion the patient was found unconscious. He had aspirated stomach contents into his lungs. His respiration, blood pressure, and pulse were all severely depressed. He never regained conciousness, and he died 5 days later. The medical examiner's finding was probable acute methadone intoxication. In this case serotonin syndrome appears to have opposed and delayed typical /CNS depression/ symptoms. Methadone has additional pharmacologic and toxicologic properties which may complicate the assessment and treatment in overdose situations.
PMID:19544673 Martinez TT, Martinez DN; Proc West Pharmacol Soc 51: 42-4 (2008)
A case of death attributed to methadone acute poisoning in an infant aged 11 months is reported. A sudden infant death syndrome (SIDS) was suspected, whereas a traumatic cause of death was excluded regarding autopsy findings. Specimens were submitted to a large toxicological analysis, which included ethanol measurement by HS-GC-FID, a targeted screening for drugs of abuse and various prescription drug classes followed by quantification using UPLC-MS/MS methods. Methadone and its metabolite (EDDP) were detected in all the tested fluids, as well as in hair, with a blood concentration of methadone considered as lethal for children (73 ng/mL). The cause of death was determined to be acute "methadone poisoning", and the manner of death was "accidental". A discussion of the case circumstances, the difficulties with the interpretation of toxicological findings in children (blood concentration and hair testing), and the origin of exposure are discussed.
PMID:24588273 Tournel G et al; J Forensic Sci 59 (5): 1436-40 (2014)
The death of 10 persons who died within days of starting a methadone maintenance program administered by general practitioners /is reported/. Their bodies were subject to a full autopsy by forensic pathologists, with a full toxicological examination. The mean starting dose had been 53 mg, which had been increased to a mean of 57 mg by the final dose. Death occurred after a mean of 3 days. The mean blood methadone concentration at death was 2.1 umol/L. ...
PMID:1288269 Drummer OH et al; Am J Forensic Med Pathol 13 (4): 346-50 (1992)
... A 14-month-old girl was found dead at home. Blood concentrations were 1071 and 148 ng/mL for methadone and 2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine (EDDP), respectively. ... A 5-month-old girl was taken to hospital in a pediatric unit for coma. Antemortem blood analysis demonstrated methadone exposure (142 ng/mL), and the baby was declared dead 12 days after admission. ... The death of the babies was attributed to accidental asphyxia ina situation where methadone was considered as a chemical weapon. The mothers, who were the perpetrators in both cases, did not deny the use of methadone as a sedative drug.
PMID:16404812 Kintz P et al; Ther Drug Monit 27 (6): 741-3 (2005)
A fatal overdose with 420 mg of oral methadone is reported in a 22-yr-old male patient known as a drug abuser and a heavy smoker and drinker who had taken the drug, which he obtained illicitly, ... .
Hendra TJ et al; Br Med J 313 (8): 481-2 (1996)
In this report, the authors present a case of unusual, accidental methadone intoxication in a 40-year-old man, who had inhaled methadone powder. ... The blood methadone concentration was 290 ug/L. The urine methadone concentration was 160 ug/L. ...
PMID:21361950 Palmiere C et al; J Forensic Sci 56 (4): 1072-5 (2011)
Methadone is indicated for the management of pain severe enough to require an opioid analgesic and for which alternative treatment options are inadequate. It's recommended that use is reserved for use in patients for whom alternative treatment options (eg, nonopioid analgesics, opioid combination products) are ineffective, not tolerated, or would be otherwise inadequate to provide sufficient management of pain. Methadone is also indicated for detoxification treatment of opioid addiction (heroin or other morphine-like drugs), and for maintenance substitution treatment for opioid dependence in adults in conjunction with appropriate social and medical services.
FDA Label
Overall, methadone's pharmacological actions result in analgesia, suppression of opioid withdrawal symptoms, sedation, miosis (through binding to receptors in the pupillary muscles), sweating, hypotension, bradycardia, nausea and vomiting (via binding within the chemoreceptor trigger zone), and constipation. Like many basic drugs, methadone also enters mast cells and releases histamine by a non-immunological mechanism leading to flushing, pruritus, and urticaria, which can commonly be misattributed to an allergic reaction. Compared to other opioids, methadone has fewer active metabolites and therefore a lower risk of neuropsychiatric toxicity. This means that higher doses needed to manage severe pain or addiction are less likely to result in delirium, hyperalgesia, or seizures. Similar to morphine, both methadone isomers are 5-HT(3) receptor antagonists, although l-methadone produces greater inhibition than d-methadone. Methadone's effects are reversible by naloxone with a pA2 value similar to its antagonism of morphine. **Dependence and Tolerance** As with other opioids, tolerance and physical dependence may develop upon repeated administration of methadone and there is a potential for development of psychological dependence. Physical dependence and tolerance reflect the neuroadaptation of the opioid receptors to chronic exposure to an opioid and are separate and distinct from abuse and addiction. Tolerance, as well as physical dependence, may develop upon repeated administration of opioids, and are not by themselves evidence of an addictive disorder or abuse. Patients on prolonged therapy should be tapered gradually from the drug if it is no longer required for pain control. Withdrawal symptoms may occur following abrupt discontinuation of therapy or upon administration of an opioid antagonist. Some of the symptoms that may be associated with abrupt withdrawal of an opioid analgesic include body aches, diarrhea, gooseflesh, loss of appetite, nausea, nervousness or restlessness, anxiety, runny nose, sneezing, tremors or shivering, stomach cramps, tachycardia, trouble with sleeping, unusual increase in sweating, palpitations, unexplained fever, weakness and yawning. **Cardiac Conduction Effects** Laboratory studies, both in vivo and in vitro, have demonstrated that methadone inhibits cardiac potassium channels and prolongs the QT interval. Cases of QT interval prolongation and serious arrhythmia (torsades de pointes) have been observed during treatment with methadone. These cases appear to be more commonly associated with, but not limited to, higher dose treatment (> 200 mg/day). Methadone should be administered with particular caution to patients already at risk for development of prolonged QT interval (e.g., cardiac hypertrophy, concomitant diuretic use, hypokalemia, hypomagnesemia). Careful monitoring is recommended when using methadone in patients with a history of cardiac conduction disease, those taking medications affecting cardiac conduction, and in other cases where history or physical exam suggest an increased risk of dysrhythmia. **Respiratory Depression and Overdose** Serious, life-threatening, or fatal respiratory depression may occur with use of methadone. Patients should be monitored for respiratory depression, especially during initiation of methadone or following a dose increase. Respiratory depression is of particular concern in elderly or debilitated patients as well as in those suffering from conditions accompanied by hypoxia or hypercapnia when even moderate therapeutic doses may dangerously decrease pulmonary ventilation. Methadone should be administered with extreme caution to patients with conditions accompanied by hypoxia, hypercapnia, or decreased respiratory reserve such as: asthma, chronic obstructive pulmonary disease or cor pulmonale, severe obesity, sleep apnea syndrome, myxedema, kyphoscoliosis, and CNS depression or coma. In these patients, even usual therapeutic doses of methadone may decrease respiratory drive while simultaneously increasing airway resistance to the point of apnea. Alternative, non-opioid analgesics should be considered, and methadone should be employed only under careful medical supervision at the lowest effective dose. Infants exposed in-utero or through breast milk are at risk of life-threatening respiratory depression upon delivery or when nursed. Methadone's peak respiratory depressant effects typically occur later, and persist longer than its peak analgesic effects, in the short-term use setting. These characteristics can contribute to cases of iatrogenic overdose, particularly during treatment initiation and dose titration. **Head Injury and Increased Intracranial Pressure** The respiratory depressant effects of opioids and their capacity to elevate cerebrospinal fluid pressure may be markedly exaggerated in the presence of head injury, other intracranial lesions or a pre-existing increase in intracranial pressure. Furthermore, opioids produce effects which may obscure the clinical course of patients with head injuries. In such patients, methadone must be used with caution, and only if it is deemed essential. **Incomplete Cross-tolerance between Methadone and other Opioids** Patients tolerant to other opioids may be incompletely tolerant to methadone. Incomplete cross-tolerance is of particular concern for patients tolerant to other -opioid agonists who are being converted to methadone, thus making the determination of dosing during opioid conversion complex. Deaths have been reported during conversion from chronic, high-dose treatment with other opioid agonists. A high degree of opioid tolerance does not eliminate the possibility of methadone overdose, iatrogenic or otherwise. Crosstolerance between morphine and methadone has been demonstrated, as steady-state plasma methadone concentrations required for effectiveness (C50%) were higher in abstinent rats previously dosed with morphine, as compared to controls. **Misuse, Abuse, and Diversion of Opioids** Methadone is a mu-agonist opioid with an abuse liability similar to morphine. Methadone, like morphine and other opioids used for analgesia, has the potential for being abused and is subject to criminal diversion. Methadone can be abused in a manner similar to other opioid agonists, legal or illicit. This should be considered when dispensing Methadone in situations where the clinician is concerned about an increased risk of misuse, abuse, or diversion. **Hypotensive Effect** The administration of methadone may result in severe hypotension in patients whose ability to maintain normal blood pressure is compromised (e.g., severe volume depletion). **Gastrointestinal Effects** Methadone and other morphine-like opioids have been shown to decrease bowel motility and cause constipation. This primarily occurs through agonism of opioid receptors in the gut wall. Methadone may obscure the diagnosis or clinical course of patients with acute abdominal conditions. **Sexual Function/Reproduction** Reproductive function in human males may be decreased by methadone treatment. Reductions in ejaculate volume and seminal vesicle and prostate secretions have been reported in methadone-treated individuals. In addition, reductions in serum testosterone levels and sperm motility, and abnormalities in sperm morphology have been reported. Long-term use of opioids may be associated with decreased sex hormone levels and symptoms such as low libido, erectile dysfunction, or infertility.
Analgesics, Opioid
Compounds with activity like OPIATE ALKALOIDS, acting at OPIOID RECEPTORS. Properties include induction of ANALGESIA or NARCOSIS. (See all compounds classified as Analgesics, Opioid.)
Antitussive Agents
Agents that suppress cough. They act centrally on the medullary cough center. EXPECTORANTS, also used in the treatment of cough, act locally. (See all compounds classified as Antitussive Agents.)
Narcotics
Agents that induce NARCOSIS. Narcotics include agents that cause somnolence or induced sleep (STUPOR); natural or synthetic derivatives of OPIUM or MORPHINE or any substance that has such effects. They are potent inducers of ANALGESIA and OPIOID-RELATED DISORDERS. (See all compounds classified as Narcotics.)
N07BC02
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
N - Nervous system
N07 - Other nervous system drugs
N07B - Drugs used in addictive disorders
N07BC - Drugs used in opioid dependence
N07BC02 - Methadone
Absorption
Methadone is one of the more lipid-soluble opioids and is well absorbed from the gastrointestinal tract. Following oral administration of methadone, bioavailability ranges from 36-100%, with a marked interindividual variation. It can be detected in blood as soon as 15-45 minutes following administration with peak plasma concentrations achieved between 1 to 7.5 hours. A second peak is observed ~4 hours after administration and is likely due to enterohepatic circulation. Dose proportionality of methadone pharmacokinetics is not known. Following administration of daily oral doses ranging from 10 to 225 mg the steady-state plasma concentrations ranged between 65 to 630 ng/mL and the peak concentrations ranged between 124 to 1255 ng/mL. Effect of food on the bioavailability of methadone has not been evaluated. Slower absorption is observed in opioid users compared to healthy subjects, which may reflect the pharmacological effect of opioids in slowing gastric emptying and mobility. Due to the large inter-individual variation in methadone pharmacokinetics and pharmacodynamics, treatment should be individualized to each patient. There was an up to 17-fold interindividual variation found in methadone blood concentrations for a given dosage, likely due in part to individual variability in CYP enzyme function. There is also a large variability in pharmacokinetics between methadone's enantiomers, which further complicates pharmacokinetic interpretation and study.
Route of Elimination
The elimination of methadone is mediated by extensive biotransformation, followed by renal and fecal excretion. Unmetabolized methadone and its metabolites are excreted in urine to a variable degree.
Volume of Distribution
Due to interindividual differences in pharmacokinetics, estimates of methadone's volume of distribution have ranged from 189-470 L with monographs listing it between 1.0-8.0L/kg. As this is higher than physiological volumes of total body water, methadone is highly distributed in the body including brain, gut, kidney, liver, muscle, and lung. A population pharmacokinetic study found that subject gender and weight explained ~33% of the variance in the apparent volume of distribution of methadone. Methadone is found to be secreted in saliva, sweat, breast milk, amniotic fluid and umbilical cord plasma. The concentration in cord blood is about half the maternal levels.
Clearance
Due to interindividual differences in pharmacokinetics, estimates of methadone's clearance have ranged from 5.913 L/h hours with approved monographs listing it between 1.4 to 126 L/h.
/MILK/ Although methadone maintenance is not a contraindication to breast feeding, it is best to avoid breast feeding 3-4 hr after the dose when peak milk levels occur. One death has been reported in a malnourished 5 week old infant whose mother was on methadone maintenance. Methadone was detected in the infant on autopsy, but it is unclear what part the drug played in the infant's death.
Knoben, J.E. and P.O. Anderson (eds.) Handbook of Clinical Drug Data. 6th ed. Bethesda, MD: Drug Intelligence Publications, Inc. 1988., p. 160
In horses, orally administered methadone appears to be well absorbed after oral administration, but bioavailability is approximately 3X lower when administered intragastrically. P-glycoprotein may paly a role in the poor intestinal absorption of methadone in vivo.
Plumb D.C. Veterinary Drug Handbook. 8th ed. (pocket). Ames, IA: Wiley-Blackwell, 2015., p. 936
In cats, after administration of methadone IV (0.3 mg/kg) or via oral transmucosal (OTM) administration (0.6 mg/kg), peak levels occurred at 10 minutes (IV) and 2 hours (OTM).
Plumb D.C. Veterinary Drug Handbook. 8th ed. (pocket). Ames, IA: Wiley-Blackwell, 2015., p. 936
Treatment of opiate use disorders with methadone is complicated by wide interindividual variability in pharmacokinetics. To identify potentially contributing covariates in methadone pharmacokinetics, we used population pharmacokinetic modeling to estimate clearance (CL/F) and volume of distribution (V/F) for each methadone enantiomer in an ethnically diverse methadone maintained population. Plasma levels of the opiate-active R-methadone and opiate-inactive S-methadone were measured in 206 methadone maintained subjects approximately two and twenty-three hours after a daily oral dose of rac-methadone. A linear one-compartment population pharmacokinetic model with first-order conditional estimation with interaction (FOCE-I) was used to evaluate methadone CL/F and V/F. The influence of covariates on parameter estimates was evaluated using stepwise covariate modeling. Covariates included ethnicity, gender, weight, BMI, age, methadone dose, and 21 single nucleotide polymorphisms in genes implicated in methadone pharmacokinetics. In the final model, for each enantiomer, Hmong ethnicity reduced CL/F by approximately 30% and the rs2032582 (ABCB1 2677G>T/A) GG genotype was associated with a 20% reduction in CL/F. The presence of the rs3745274 minor allele (CYP2B6 515G>T) reduced CL/F by up to 20% for S-methadone only. A smaller effect of age was noted on CL/F for R-methadone. This is the first report showing the influence of the rs2032582 and rs3745274 variants on methadone pharmacokinetics rather than simply dose requirements or plasma levels. Population pharmacokinetics is a valuable method for identifying the influences on methadone pharmacokinetic variability.
PMID:25456329 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4254688 Bart G et al; Drug Alcohol Depend 145: 185-93 (2014)
For more Absorption, Distribution and Excretion (Complete) data for Methadone (14 total), please visit the HSDB record page.
Methadone undergoes fairly extensive first-pass metabolism. Cytochrome P450 enzymes, primarily CYP3A4, CYP2B6, and CYP2C19 and to a lesser extent CYP2C9, CYP2C8, and CYP2D6, are responsible for conversion of methadone to EDDP (2-ethyl-1,5-dimethyl-3,3-diphenylpyrrolidine) and other inactive metabolites, which are excreted mainly in the urine. Methadone first undergoes N-demethylation to form a highly unstable compound that spontaneously converts to EDDP through cyclization and dehydration. EDDP is then converted to 2-ethyl5-methyl-3,3-diphenyl-1-pyrroline (EDMP). Both EDDP and EDMP are inactive. The CYP isozymes also demonstrate different affinities for metabolizing the different methadone enantiomers: CYP2C19, CYP3A7, and CYP2C8 preferentially metabolize (R)-methadone while CYP2B6, CYP2D6, and CYP2C18 preferentially metabolize (S)-methadone. CYP3A4 does not have an enantiomer preference. Single nucleotide polymorphisms (SNPs) within the cytochrome P450 enzymes can impact methadone pharmacokinetics and contribute to the interindividual variation in response to methadone therapy. In particular, CYP2B6 polymorphisms have been shown to impact individual response to methadone as it is the predominant determinant involved in the N-demethylation of methadone, clearance, and the metabolic ratios of [methadone\]/[EDDP]. The SNPs CYP2B6\*6, \*9, \*11, CYP2C19\*2, \*3, CYP3A4\*1B, and CYP3A5\*3 result in increased methadone plasma concentrations, decreased N-demethylation, and decreased methadone clearance, while homozygous carriers of CYP2B6\*6/\*6 demonstrate diminished metabolism and clearance of methadone. See the pharmacogenomics section for further information. Pharmacogenomic effects on the CYP enzymes can be significant as the long half-life of methadone can result in some individuals having higher than normal therapeutic levels which puts them at risk of dose-related side effects. For example, elevated (R)-methadone levels can increase the risk of respiratory depression, while elevated (S)-methadone levels can increase the risk of severe cardiac arrhythmias due to prolonged QTc interval.
... The drug/metabolite concentrations and ratios of methadone to two of its metabolites (2-ethylidene-1, 5-dimethyl-3, 3-diphenylpyrrolidine; and 2-ethyl-5-methyl-3,3-diphenylpyrroline) in postmortem peripheral blood and liver tissue by liquid chromatography/tandem mass spectrometry /were determined/. The assays employed deuterated internal standards and multiple reaction monitoring (MRM) techniques. The assay linear range was 0.01-2.0 mg/L for each analyte. Methadone, 2-ethylidene-1, 5-dimethyl-3, 3-diphenylpyrrolidine, and 2-ethyl-5-methyl-3,3-diphenylpyrroline were determined in liver and peripheral blood from 46 methadone-positive cases. Methadone and 2-ethylidene-1, 5-dimethyl-3, 3-diphenylpyrrolidine were detected in all specimens, whether blood or liver. 2-ethyl-5-methyl-3,3-diphenylpyrroline was detected, only in liver, and only 17 cases, at concentrations much lower than those of 2-ethylidene-1, 5-dimethyl-3, 3-diphenylpyrrolidine. Concentrations of methadone and 2-ethylidene-1, 5-dimethyl-3, 3-diphenylpyrrolidine in blood and liver from 2-ethyl-5-methyl-3,3-diphenylpyrroline-positive cases were in ranges higher than, but overlapping with, concentrations in blood and liver from 2-ethyl-5-methyl-3,3-diphenylpyrroline-negative cases. These data suggest that although methadone is readily demethylated and cyclized to 2-ethylidene-1, 5-dimethyl-3, 3-diphenylpyrrolidine, in vivo, conversion to 2-ethyl-5-methyl-3,3-diphenylpyrroline may be less efficient and its accumulation in postmortem tissues may be highly individual.
PMID:19291457 Danielson TJ et al; Forensic Sci Med Pathol 4 (3): 170-4 (2008)
Methadone is extensively metabolized, principally by cytochrome P-450 (CYP) isoenzyme 3A4 in the liver and/or intestine, although other isoenzymes, including CYP2B6, CYP1A2, and CYP2D6, also may be involved. The drug undergoes N-demethylation to an inactive metabolite, 2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidene (EDDP), and other metabolites with little or no pharmacologic activity. Although methadone appears to be a substrate of the P-glycoprotein transport system, its pharmacokinetics do not appear to be substantially altered by P-glycoprotein polymorphism or inhibition.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 2264
Metabolism is via hepatic CYP3A4 N-demethylation, with excretion of the parent (21%) and metabolites in the urine. Smaller amounts are also detectable in the bile, feces, and sweat.
Dart, R.C. (ed). Medical Toxicology. Third Edition, Lippincott Williams & Wilkins. Philadelphia, PA. 2004., p. 767
The objective of this study was to identify the enzyme that metabolizes methadone in preterm placentas. Microsomal fractions were obtained from preterm (17 to 34 weeks) placentas (36 total; 12 per each gestational age group) and their activity in metabolizing methadone to 2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine (EDDP) was determined. The enzyme catalyzing the reaction was identified by using chemical inhibitors selective for various cytochrome P450 isozymes and monoclonal antibodies raised against them. The metabolism of methadone by microsomes revealed saturation kinetics. Methadone was N-demethylated to EDDP by aromatase. The affinity of methadone to aromatase (apparent Km) did not change with gestation, but the activity of the enzyme (Vmax) increased and varied widely between individual placentas. Aromatase/CYP19 is the placental enzyme metabolizing methadone during pregnancy. The variability in enzyme activity among individuals should be reflected by the concentration of methadone in the fetal circulation and might be one of the factors affecting the incidence and intensity of neonatal abstinence syndrome.
PMID:16799915 Hieronymus TL et al; Am J Perinatol 23 (5): 287-94 (2006)
Methadone has known human metabolites that include 2-ethylidene-1,5-dimethyl-3,3-diphenylpyrolidine.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
Due to interindividual differences in pharmacokinetics, estimates of methadone's half-life have ranged from 15207 hours with official monographs listing it between 7-59 hours.
In goats, methadone has a high bioavailability after SC administration; half life is approximately 1.5 hours.
Plumb D.C. Veterinary Drug Handbook. 8th ed. (pocket). Ames, IA: Wiley-Blackwell, 2015., p. 936
Methadone is rapidly eliminated (half life around 1 hour) in horses.
Plumb D.C. Veterinary Drug Handbook. 8th ed. (pocket). Ames, IA: Wiley-Blackwell, 2015., p. 936
Terminal elimination half life after intravenous dosing is approximately 1.75-4 hours in dogs ... . In SC administration , half life is closer to 11 hours, but there was wide inter-patient variation.
Plumb D.C. Veterinary Drug Handbook. 8th ed. (pocket). Ames, IA: Wiley-Blackwell, 2015., p. 936
In clinical studies, the terminal elimination half-life of methadone ranged from 8-59 hours. In clinical use, the elimination half-life of methadone has varied considerably, ranging from 9-87 hours in postoperative patients, from 8.5-75 hours in opiate-dependent patients, and up to 120 hours in outpatients receiving therapy for chronic malignant pain. In one study in 5 patients receiving 100 or 120 mg of oral methadone hydrochloride daily for maintenance treatment of opiate addiction, the drug had an apparent plasma half-life of 13-47 hours, with an average of 25 hours.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 2264
The steady-state half life of elimination is 23 hours.
Dart, R.C. (ed). Medical Toxicology. Third Edition, Lippincott Williams & Wilkins. Philadelphia, PA. 2004., p. 767
Methadone is a synthetic opioid analgesic with full agonist activity at the -opioid receptor. While agonism of the -opioid receptor is the primary mechanism of action for the treatment of pain, methadone also acts as an agonist of - and -opioid receptors within the central and peripheral nervous systems. Interestingly, methadone differs from [morphine] (which is considered the gold standard reference opioid) in its antagonism of the N-methyl-D-aspartate (NMDA) receptor and its strong inhibition of serotonin and norepinephrine uptake, which likely also contributes to its antinociceptive activity. Methadone is administered as a 50:50 racemic mixture of (R)- and (S)-stereoisomers, with (R)-methadone demonstrating ~10-fold higher affinity and potency for the -opioid receptor than the (S) stereoisomer. The analgesic activity of the racemate is almost entirely due to the (R)-isomer, while the (S)-isomer lacks significant respiratory depressant activity but does have antitussive effects. While methadone shares similar effects and risks of other opioids such as [morphine], [hydromorphone], [oxycodone], and [fentanyl] it has a number of unique pharmacokinetic and pharmacodynamic properties that distinguish it from them and make it a useful agent for the treatment of opioid addiction. For example, methadone abstinence syndrome, although qualitatively similar to that of morphine, differs in that the onset is slower, the course is more prolonged, and the symptoms are less severe.
Methadone hydrochloride is a mu-agonist; ... . Some data also indicate that methadone acts as an antagonist at the N-methyl-D-aspartate (NMDA) receptor. The contribution of NMDA receptor antagonism to methadone's efficacy is unknown.
NIH; DailyMed. Current Medication Information for Methadone Hydrochloride Tablet (Updated: April 2017). Available from, as of October 2, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=eddf7077-02fb-4771-9823-31984f4ff2bb
Methadone activates opioid receptors to increase a potassium conductance mediated by G-protein coupled, inwardly rectifying, potassium (K(IR) 3) channels. Methadone also blocks K(IR) 3 channels and NMDA receptors. However, the concentration dependence and stereospecificity of receptor activation and channel blockade by methadone on single neurons has not been characterized. Intracellular and whole cell recording were made from locus coeruleus neurons in brain slices and the activation of mu-opioid receptors and blockade of K(IR) 3 and NMDA channels with l- and d-methadone was examined. The potency of l-methadone, measured by the amplitude of hyperpolarization was 16.5-fold higher than with than d-methadone. A maximum hyperpolarization was caused by both enantiomers (~30 mV), however, the maximum outward current measured with whole cell voltage-clamp recording was smaller than the current induced by [Met](5) enkephalin. The K(IR) 3 conductance induced by activation of a(2) -adrenoceptors was decreased with high concentrations of l- and d-methadone (10-30 uM). In addition, methadone blocked the resting inward rectifying conductance (K(IR) ). Both l- and d-methadone blocked the NMDA receptor-dependent current. The block of NMDA receptor-dependent current was voltage dependent suggesting that methadone acted as a channel blocker. Methadone activated mu-opioid receptors at low concentrations in a stereospecific manner. K(IR) 3 and NMDA receptor channel block was not stereospecific and required substantially higher concentrations. The separation in the concentration range suggests that the activation of mu-opioid receptors rather than the channel blocking properties mediate both the therapeutic and toxic actions of methadone.
PMID:20659105 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3000663 Matsui A, Williams JT; Br J Pharmacol 161 (6): 1403-13 (2010)
Opioid agonist, analgesic. Action of methadone is to bind to mu-opiate and kappa-opiate receptors on nerves and inhibit release of neurotransmitters involved with transmission of pain stimuli (such as Substance P). Methadone also may antagonize NMDA (n-methyl D-asparate) receptors, which may contribute to the analgesic effect, decrease adverse CNS effects, and inhibit tolerance. ...
Papich, M.G. Saunders Handbook of Veterinary Drugs Small and Large Animal. 3rd ed. St. Louis, MO: Elsevier Saunders, 2011, p. 483

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
61
PharmaCompass offers a list of Methadone API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Methadone manufacturer or Methadone supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Methadone manufacturer or Methadone supplier.
PharmaCompass also assists you with knowing the Methadone API Price utilized in the formulation of products. Methadone API Price is not always fixed or binding as the Methadone Price is obtained through a variety of data sources. The Methadone Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Methadone manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Methadone, including repackagers and relabelers. The FDA regulates Methadone manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Methadone API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Methadone manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Methadone supplier is an individual or a company that provides Methadone active pharmaceutical ingredient (API) or Methadone finished formulations upon request. The Methadone suppliers may include Methadone API manufacturers, exporters, distributors and traders.
click here to find a list of Methadone suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Methadone DMF (Drug Master File) is a document detailing the whole manufacturing process of Methadone active pharmaceutical ingredient (API) in detail. Different forms of Methadone DMFs exist exist since differing nations have different regulations, such as Methadone USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Methadone DMF submitted to regulatory agencies in the US is known as a USDMF. Methadone USDMF includes data on Methadone's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Methadone USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Methadone suppliers with USDMF on PharmaCompass.
Methadone Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Methadone GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Methadone GMP manufacturer or Methadone GMP API supplier for your needs.
A Methadone CoA (Certificate of Analysis) is a formal document that attests to Methadone's compliance with Methadone specifications and serves as a tool for batch-level quality control.
Methadone CoA mostly includes findings from lab analyses of a specific batch. For each Methadone CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Methadone may be tested according to a variety of international standards, such as European Pharmacopoeia (Methadone EP), Methadone JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Methadone USP).

