



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
0
Listed Suppliers
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
Uploaded Dossiers
0
U.S. Medicaid
Annual Reports
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
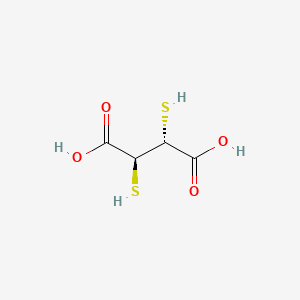

1. 2,3 Dimercaptosuccinic Acid
2. 2,3-dimercaptosuccinic Acid
3. Acid, 2,3-dimercaptosuccinic
4. Acid, Dimercaptosuccinic
5. Acid, Meso-dimercaptosuccinic
6. Butanedioic Acid, 2,3-dimercapto-, (r*,s*)-isomer
7. Chemet
8. Dimercaptosuccinate, Tin
9. Dimercaptosuccinic Acid
10. Dipotassium Salt Succimer
11. Disodium Salt Succimer
12. Dmsa
13. Meso Dimercaptosuccinic Acid
14. Meso-dimercaptosuccinic Acid
15. Monosodium Salt Succimer
16. Rhenium Salt Succimer
17. Ro 1 7977
18. Ro-1-7977
19. Ro17977
20. Succicaptal
21. Succimer Antimony Sodium Salt, (r*,s*)-isomer
22. Succimer, (r*,r*)-(+,-)-isomer
23. Succimer, Dipotassium Salt
24. Succimer, Disodium Salt
25. Succimer, Monosodium Salt
26. Succimer, Rhenium Salt
27. Succimer, Tin Salt
28. Tin Dimercaptosuccinate
29. Tin Salt Succimer
1. Meso-2,3-dimercaptosuccinic Acid
2. 304-55-2
3. Dim-sa
4. Chemet
5. Dmsa
6. Meso-dimercaptosuccinic Acid
7. (2r,3s)-rel-2,3-dimercaptosuccinic Acid
8. Succimero
9. Succimerum
10. Butanedioic Acid, 2,3-dimercapto-, (2r,3s)-rel-
11. Dimercaptosuccinic Acid
12. Ro 1-7977
13. Dms-a
14. (r*,s*)-2,3-dimercaptobutanedioic Acid
15. Butanedioic Acid, 2,3-dimercapto-, (r*,s*)-
16. Succinic Acid, 2,3-dimercapto-, Meso-
17. Dx1u2629qe
18. Chebi:63623
19. Meso-2,3-dimercaptobernsteinsaeure
20. Meso-2,3-dimercaptosuccinic Acid;
21. (2r,3s)-2,3-disulfanylsuccinic Acid
22. Succimerum [inn-latin]
23. Succimero [inn-spanish]
24. (2r,3s)-2,3-dimercaptosuccinic Acid
25. Succimer [usan:inn:ban]
26. Hsdb 6783
27. 3-03-00-01033 (beilstein Handbook Reference)
28. Einecs 206-155-2
29. Brn 1725150
30. Unii-dx1u2629qe
31. Chemet (tn)
32. Mfcd00064799
33. Dimercaptosuccinic-acid
34. Succimer [hsdb]
35. Succimer [usan]
36. Succimer (usan/inn)
37. Succimer [inn]
38. Succimer [ii]
39. Succimer [mi]
40. Succimer [vandf]
41. (2s,3r)-2,3-bis(sulfanyl)butanedioic Acid
42. Succimer [mart.]
43. Succimer [who-dd]
44. Schembl14941
45. Spectrum1505007
46. Succimer [orange Book]
47. Chembl1201073
48. Dtxsid1023601
49. 2,3-bis-sulfanylbutanedioic Acid
50. Hy-b1768
51. Zinc3831475
52. S4692
53. Akos015892684
54. Ac-1366
55. Ccg-214189
56. Db00566
57. Ds-7410
58. Dmsa (meso-2,3-dimercaptosuccinic Acid)
59. Meso-2,3-dimercaptosuccinic Acid, ~98%
60. 2,3-dimercaptosuccinic Acid, Meso-
61. Cs-0013797
62. D1722
63. A17093
64. C07598
65. D00572
66. 304d552
67. A820382
68. Q423814
69. Q-201748
70. Brd-k88701661-001-01-1
| Molecular Weight | 182.2 g/mol |
|---|---|
| Molecular Formula | C4H6O4S2 |
| XLogP3 | 0.1 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 3 |
| Exact Mass | 181.97075102 g/mol |
| Monoisotopic Mass | 181.97075102 g/mol |
| Topological Polar Surface Area | 76.6 Ų |
| Heavy Atom Count | 10 |
| Formal Charge | 0 |
| Complexity | 139 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Chemet |
| PubMed Health | Succimer (By mouth) |
| Drug Classes | Heavy Metal Chelator |
| Drug Label | CHEMET (succimer) is an orally active, heavy metal chelating agent. The chemical name for succimer is meso 2, 3-dimercaptosuccinic acid (DMSA). Its empirical formula is C4H6O4S2 and molecular weight is 182.2. The meso-structural formula is:Succimer i... |
| Active Ingredient | Succimer |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 100mg |
| Market Status | Prescription |
| Company | Recordati Rare |
| 2 of 2 | |
|---|---|
| Drug Name | Chemet |
| PubMed Health | Succimer (By mouth) |
| Drug Classes | Heavy Metal Chelator |
| Drug Label | CHEMET (succimer) is an orally active, heavy metal chelating agent. The chemical name for succimer is meso 2, 3-dimercaptosuccinic acid (DMSA). Its empirical formula is C4H6O4S2 and molecular weight is 182.2. The meso-structural formula is:Succimer i... |
| Active Ingredient | Succimer |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 100mg |
| Market Status | Prescription |
| Company | Recordati Rare |
Antidotes; Chelating Agents
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
No controlled clinical studies have been conducted with succimer in poisoning with other heavy metals. A limited number of patients have received succimer for mercury or arsenic poisoning. These patients showed increased urinary excretion of the heavy metal and varying degrees of symptomatic improvement. /Use NOT included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for CHEMET (succimer) capsule (May 2010). Available from, as of June 23, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=18210
Chemet is indicated for the treatment of lead poisoning in pediatric patients with blood lead levels above 45 ug/dL. Chemet is not indicated for prophylaxis of lead poisoning in a lead-containing environment; the use of Chemet should always be accompanied by identification and removal of the source of the lead exposure. /Use included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for CHEMET (succimer) capsule (May 2010). Available from, as of June 23, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=18210
Orphan Drug. Drug (Trade Name): Succimer (Chemet). Proposed Use: Prevent cystine kidney stones in patients with homozygous cystinuria who are prone to stone development; mercury intoxication. /From table/
Novak, K.M. (ed.). Drug Facts and Comparisons2008 Edition. Wolters Kluwer Health. St. Louis, Missouri 2008., p. KU-32
For more Therapeutic Uses (Complete) data for Succimer (10 total), please visit the HSDB record page.
It is not known whether this drug is excreted in human milk. Because many drugs and heavy metals are excreted in human milk, nursing mothers requiring Chemet therapy should be discouraged from nursing their infants.
US Natl Inst Health; DailyMed. Current Medication Information for CHEMET (succimer) capsule (May 2010). Available from, as of June 23, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=18210
Elevated blood lead levels and associated symptoms may return rapidly after discontinuation of CHEMET because of redistribution of lead from bone stores to soft tissues and blood. After therapy, patients should be monitored for rebound of blood lead levels, by measuring blood lead levels at least once weekly until stable. However, the severity of lead intoxication (as measured by the initial blood lead level and the rate and degree of rebound of blood lead) should be used as a guide for more frequent blood lead monitoring.
US Natl Inst Health; DailyMed. Current Medication Information for CHEMET (succimer) capsule (May 2010). Available from, as of June 23, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=18210
All patients undergoing treatment should be adequately hydrated. Caution should be exercised in using Chemet therapy in patients with compromised renal function. Limited data suggests that Chemet is dialyzable, but that the lead chelates are not.
US Natl Inst Health; DailyMed. Current Medication Information for CHEMET (succimer) capsule (May 2010). Available from, as of June 23, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=18210
The possibility of allergic or other mucocutaneous reactions to the drug must be borne in mind on readministration (as well as during initial courses). Patients requiring repeated courses of Chemet should be monitored during each treatment course. One patient experienced recurrent mucocutaneous vesicular eruptions of increasing severity affecting the oral mucosa, the external urethral meatus and the perianal area on the third, fourth and fifth courses of the drug. The reaction resolved between courses and upon discontinuation of therapy.
US Natl Inst Health; DailyMed. Current Medication Information for CHEMET (succimer) capsule (May 2010). Available from, as of June 23, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=18210
For more Drug Warnings (Complete) data for Succimer (11 total), please visit the HSDB record page.
For the treatment of lead poisoning in pediatric patients with blood lead levels above 45 µg/dL. May also be used to treat mercury or arsenic poisoning.
Succimer is an orally active, heavy metal chelating agent. It forms water soluble chelates and, consequently, increases the urinary excretion of lead. Succimer is not to be used for prophylaxis of lead poisoning in a lead-containing environment. In addition, the use of succimer should always be accompanied by identification and removal of the source of the lead exposure.
Antidotes
Agents counteracting or neutralizing the action of POISONS. (See all compounds classified as Antidotes.)
Chelating Agents
Chemicals that bind to and remove ions from solutions. Many chelating agents function through the formation of COORDINATION COMPLEXES with METALS. (See all compounds classified as Chelating Agents.)
Absorption
Rapid but variable.
Route of Elimination
Unabsorbed drug is excreted primarily in feces and absorbed drug is excreted primarily in the urine as metabolites.
In a study performed in healthy adult volunteers, after a single dose of (14)Csuccimer at 16, 32, or 48 mg/kg, absorption was rapid but variable with peak blood radioactivity levels between one and two hours. On average, 49% of the radiolabeled dose was excreted: 39% in the feces, 9% in the urine and 1% as carbon dioxide from the lungs. Since fecal excretion probably represented nonabsorbed drug, most of the absorbed drug was excreted by the kidneys. The apparent elimination half-life of the radiolabeled material in the blood was about two days.
US Natl Inst Health; DailyMed. Current Medication Information for CHEMET (succimer) capsule (May 2010). Available from, as of June 23, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=18210
In other studies of healthy adult volunteers receiving a single oral dose of 10 mg/kg, the chemical analysis of succimer and its metabolites in the urine showed that succimer was rapidly and extensively metabolized. Approximately 25% of the administered dose was excreted in the urine with the peak blood level and urinary excretion occurring between two and four hours. Of the total amount of drug eliminated in the urine, approximately 90% was eliminated in altered form as mixed succimer-cysteine disulfides; the remaining 10% was eliminated unchanged. The majority of mixed disulfides consisted of succimer in disulfide linkages with two molecules of L-cysteine, the remaining disulfides contained one L-cysteine per succimer molecule.
US Natl Inst Health; DailyMed. Current Medication Information for CHEMET (succimer) capsule (May 2010). Available from, as of June 23, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=18210
The urinary excretion of succimer (meso-2,3-dimercaptosuccinic acid) was studied following oral administration of 10 mg succimer/kg to 6 normal men, aged 22-31 yr. The succimer that was absorbed was extensively biotransformed. After 14 hr only 2.53% of the drug was excreted in the urine as unaltered succimer and 18.1% as altered forms. The unaltered succimer was 12% of the total succimer found in the urine. The altered form(s) of succimer was 88% of the total urinary succimer. The altered succimer can be converted to unaltered succimer by electrolytic reduction, which indicates that the altered forms of succimer are disulfides. The excretion of altered succimer reached a peak between 2 and 4 hr after administration. There were small but significant increases in the excretion of zinc, copper, and lead after succimer. The chelating agent did not influence the urinary excretion of 27 other metals and elements.
PMID:2541962 Aposhian HV et al; Clin Pharmacol Ther 45 (May): 520-6 (1989)
(14)C DMSA was administered to mice iv; the mice were frozen by immersion in dry ice/hexane at 6 and 20 min and 1, 3, 9, and 24 hr after injection. The frozen mice were sectioned and processed for whole-body autoradiography for soluble substances. The radioactivity was highly localized in extracellular fluids such as the sc, intrapleural, ip, and periosteal spaces. There was a pronounced accumulation in the periosteal fluid above that in other fluids during the first hour after injection. Most of the radioactivity was eliminated by the kidney and liver. Pretreatment of a mouse with HgCl2 subcutaneously 1 hr before (14)C DMSA produced an increase in radioactivity in the liver and decrease in lung. A high concentration of radioactivity was seen at the sc site of injection of the HgCl2. The results are interpreted to indicate that most of the DMSA is in the extracellular space but that it can cross cellular membranes to some extent. The pronounced accumulation in periosteal fluid may be an interaction of DMSA with Ca2+ in this space. No tissue had a pronounced retention of the compound, but lung retained more than most other tissues.
PMID:3009254 Lang YY et al; Fundam Appl Toxicol 6 (3): 532-40 (1986)
Chemical analysis of succimer and its metabolites (primarily mixed disulfides of L-cysteine) in the urine showed that succimer was rapidly and extensively metabolized however the specific site of biotransformation is not known.
/Two/ subjects were given DMSA at 10 mg/kg orally, and urine samples were collected at 1, 2, 4, 6, 9 and 14 hr after administration. Samples were analyzed by HPLC, ion exchange, and TLC techniques. Most of the ingested DMSA was found in the urine in disulfide linkage with L-cysteine. Electrolytic reductive treatment, which breaks disulfide bonds, resulted in the conversion of the mixed disulfides to DMSA and L-cysteine. After the sulfhydryl group was derivatized and the bimane derivatives analyzed by HPLC and fluorescence, a high correlation between the excretion of L-cysteine and DMSA in the urine was evident. Four of the metabolites found in the urine contained L-cysteine and DMSA in different ratios. Results indicated that when DMSA is given orally to humans, it forms mixed disulfides with L-cysteine in preference to the formation of cyclic disulfides of DMSA. L-Cysteine preferentially forms these unique DMSA/mixed disulfides instead of the usual L-cystine. The amount of L-cysteine excreted in the urine as the mixed disulfides far exceeded the amount excreted as L-cystine. This increased excretion of L-cysteine caused by the DMSA exposure implied that a thiol-disulfide exchange between L-cystine and DMSA occurred and/or a direct reaction between L-cysteine and DMSA occurred to form more soluble mixed disulfides.
PMID:2538007 Maiorino RM et al; Toxicol Appl Pharmacol 97 (2): 338-49 (1989)
48 hours
The apparent elimination half-life of the radiolabeled material in the blood was about two days.
US Natl Inst Health; DailyMed. Current Medication Information for CHEMET (succimer) capsule (May 2010). Available from, as of June 23, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=18210
Succimer is a heavy metal chelator. It binds with high specificity to ions of lead in the blood to form a water-soluble complex that is subsequently excreted by the kidneys. Succimer can also chelate mercury, cadmium, and arsenic in this manner.
Succimer is a lead chelator; it forms water soluble chelates and, consequently, increases the urinary excretion of lead.
US Natl Inst Health; DailyMed. Current Medication Information for CHEMET (succimer) capsule (May 2010). Available from, as of June 23, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=18210
DMSA chelates by coordination of one sulfur and one oxygen atom with Pb. Solubility of the lead chelates depends on the ionization of the noncoordinated thiol and carboxylic acid groups. Bimane derivatization, HPLC, and fluorescence, as well as gas chromatography can be used for analysis of DMSA in biological fluids. The acid dissociation constants for meso- and racemic-DMSA have been summarized from the literature as have the formation constants of some of the DMSA chelates. DMSA is biotransformed to a mixed disulfide in humans. By 14 hr after DMSA administration (10 mg/kg), only 2.5% of the administered DMSA is excreted in the urine as unaltered DMSA and 18.1% of the dose is found in the urine as altered forms of DMSA. Most altered DMSA in the urine is in the form of a mixed disulfide. It consists of DMSA in disulfide linkages with two molecules of L-cysteine. One molecule of cysteine is attached to each of the sulfur atoms of DMSA. The remaining 10% of the altered DMSA was in the form of cyclic disulfides of DMSA. So far, the mixed disulfide has been found in human but not in rabbit, mouse, or rat urine. Apparently there are species differences in how organisms metabolize meso-DMSA.
PMID:2160791 Aposhian HV, Aposhian MM; Annu Rev Pharmacol Toxicol 30: 279-306 (1990)


