
Synopsis
Synopsis
0
JDMF
0
KDMF
0
VMF
0
Canada
0
Australia
0
US Patents
0
US Exclusivities
0
Health Canada Patents
Annual Reports
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz

1. Dapaz
2. Equanil
3. Mprobamate Richard
4. Meprospan
5. Miltown
6. Tranmep
7. Visano
1. Equanil
2. Meprospan
3. Miltown
4. Meprobamat
5. Tranmep
6. Amepromat
7. Meprocompren
8. Neuramate
9. 57-53-4
10. Amosene
11. Mepriam
12. Meprobam
13. Meproban
14. Dapaz
15. Anastress
16. Anathylmon
17. Anatimon
18. Andaksin
19. Ansiatan
20. Ansiowas
21. Anxietil
22. Auxietil
23. Ayeramate
24. Biobamat
25. Biobamate
26. Calmadin
27. Calmiren
28. Carbaxin
29. Cirponyl
30. Crestanil
31. Despasmol
32. Dicandiol
33. Equatrate
34. Equilium
35. Harmonin
36. Holbamate
37. Ipsotian
38. Lepetown
39. Libiolan
40. Margonil
41. Mepantin
42. Mepavlon
43. Mepiosine
44. Mepranil
45. Meprindon
46. Meprocon
47. Meprodil
48. Meproleaf
49. Meprosan
50. Meprosin
51. Meprotabs
52. Meprotan
53. Meprotil
54. Metractyl
55. Metranquil
56. Miltamato
57. Andaxin
58. Aneural
59. Anural
60. Apascil
61. Arcoban
62. Artolon
63. Atraxin
64. Calmax
65. Cirpon
66. Cyrpon
67. Ecuanil
68. Edenal
69. Enorden
70. Epicur
71. Epikur
72. Equitar
73. Estasil
74. Gadexyl
75. Hartol
76. Larten
77. Lepenil
78. Mendel
79. Meposed
80. Meprin
81. Meprol
82. Meprosa
83. Meptran
84. Milprem
85. Miltaun
86. Ansil
87. Anzil
88. Arpon
89. Diron
90. Erina
91. Klort
92. Letyl
93. Kesso-bamate
94. Appetrol-sr
95. Meprocon Cmc
96. Mar-bate
97. Appetrol
98. Dormabrol
99. Meprodiol
100. Micrainin
101. Bamate
102. Deprol
103. Diurnal
104. Diveron
105. Milpath
106. 3p Bamate
107. Meprovanmeprozine
108. Canquil-400
109. Equanil Suspension
110. Meptranactylmilprem
111. Procalmidol
112. Procarbamide
113. Fas-cile 200
114. Cap-o-tran
115. Meprobamat [german]
116. Bamo 400
117. Diurnaldiverondormabrol
118. Meprobamato [italian]
119. Procalmadiol
120. Aneusral
121. Aneuxral
122. Ataraxine
123. Ayermate
124. Brobamate
125. Coprobate
126. Mepamtin
127. Meprobamato
128. Miltrate
129. Misedant
130. Nephentine
131. Nervonus
132. Optarket
133. Orolevol
134. Pancalma
135. Panediol
136. Pankalma
137. Perequietil
138. Perequil
139. Perquietil
140. Pertranquil
141. Placidon
142. Placitate
143. Probamato
144. Probamyl
145. Procalmadol
146. Proquanil
147. Quietidon
148. Rastenil
149. Reostral
150. Restenil
151. Restenyl
152. Restinal
153. Restinil
154. Robamate
155. Scolazil
156. Sedabamate
157. Sedoquil
158. Sedoselecta
159. Shalvaton
160. Spantran
161. Stensolo
162. Tensonal
163. Trankvilan
164. Tranlisant
165. Tranquilan
166. Tranquilate
167. Tranquilax
168. Tranquiline
169. Tranquilsan
170. Tranquinol
171. Vistabamate
172. Wardamate
173. Aneurol
174. Aneuxal
175. Apasil
176. Cypron
177. Equinil
178. Gagexyl
179. Miltann
180. Miltuan
181. Miltwon
182. Morbam
183. Multaun
184. Orlevol
185. Pensive
186. Prequil
187. Promate
188. Promato
189. Protran
190. Quaname
191. Quanane
192. Quanil
193. Quivet
194. Sadanyl
195. Sedanil
196. Sedanyl
197. Sedazil
198. Selene
199. Setran
200. Sowell
201. Tamate
202. Tensol
203. Trelmar
204. Urbilat
205. Wyseals
206. Zirpon
207. Oasil
208. Paxin
209. Pimal
210. Seril
211. Urbil
212. Meprobamic Acid
213. Pan-tranquil
214. Meprobamatum [inn-latin]
215. Vio-bamate
216. Meprobamato [inn-spanish]
217. Fas-cile
218. Neo-tran
219. Sk-bamate
220. Solevione Anastress
221. 2,2-di(carbamoyloxymethyl)pentane
222. Bamd 400
223. 1,3-propanediol, 2-methyl-2-propyl-, Dicarbamate
224. 2-methyl-2-propyl-1,3-propanediol Dicarbamate
225. Dea No. 2820
226. Meprobamate Civ
227. 2-methyl-2-propyltrimethylene Carbamate
228. Pmb-200
229. Pmb-400
230. 2-methyl-2-n-propyl-1,3-propanediol Dicarbamate
231. [2-(carbamoyloxymethyl)-2-methylpentyl] Carbamate
232. 2-[(carbamoyloxy)methyl]-2-methylpentyl Carbamate
233. Carbamic Acid, 2-methyl-2-propyltrimethylene Ester
234. Meprotanum
235. Nsc-30418
236. Mepro-analgesic
237. Carbamic Acid 2-methyl-2-propyltrimethylene Ester
238. Equazine-m
239. 9i7lny769q
240. Pathibamate
241. 1,3-propanediol, 2-methyl-2-propyl-, 1,3-dicarbamate
242. Meprospan-200
243. Meprospan-400
244. Meprin (van)
245. Ncgc00091031-01
246. Kessobamate
247. Meprobamatum
248. Ansietan
249. Meproten
250. Meprovan
251. Miltown 600
252. My-trans
253. Dsstox_cid_3261
254. Dsstox_rid_76946
255. Dsstox_gsid_23261
256. Canquil 400
257. Meprobamate And Aspirin Tablets
258. Cas-57-53-4
259. Carb-a-med
260. Equanil (tn)
261. Miltown (tn)
262. Component Of Milpath
263. Component Of Milprem
264. Tranquiline (intra)
265. Component Of Appetrol
266. Component Of Miltrate
267. Component Of Equalysen
268. Hsdb 3117
269. Component Of Pmb-400
270. Einecs 200-337-5
271. Brn 1788882
272. Unii-9i7lny769q
273. Microbamat
274. Meprobamate (jan/usp/inn)
275. Mepr
276. 2-metil-2-n-propil-1,3-propanediol Dicarbamato [spanish]
277. Meprobamate [usp:inn:ban:jan]
278. 2-metil-2-n-propil-1,3-propanediol Dicarbamato
279. Meprobamat-petrasch
280. Pathibamate (salt/mix)
281. Meprobamate [mi]
282. Meprobamate [inn]
283. Meprobamate [jan]
284. Meprobamate [hsdb]
285. Chembl979
286. Meprobamate [vandf]
287. Meprobamate [mart.]
288. Schembl15286
289. Meprobamate [who-dd]
290. Mls003899229
291. Chebi:6761
292. Gtpl7225
293. Meprobamate, Analytical Standard
294. Component Of Deprol (salt/mix)
295. Dtxsid3023261
296. Component Of Milpath (salt/mix)
297. Component Of Milprem (salt/mix)
298. Wln: Zvo1x3&1&1ovz
299. Meprobamate [ep Impurity]
300. Meprobamate [orange Book]
301. Meprobamate Civ [usp-rs]
302. Component Of Appetrol (salt/mix)
303. Component Of Miltrate (salt/mix)
304. Meprobamate [usp Monograph]
305. Meprobamate 1.0 Mg/ml In Methanol
306. Nsc30418
307. Zinc1530701
308. Component Of Pmb-400 (salt/mix)
309. Q-gesic Component Meprobamate
310. Tox21_111063
311. Tox21_200332
312. Nsc 30418
313. 1, 2-methyl-2-propyl-, Dicarbamate
314. Equagesic Component Meprobamate
315. Micrainin Component Meprobamate
316. Akos003617983
317. Db00371
318. Carisoprodol Ep Impurity D; Meprobamate
319. Meprobamate Component Of Q-gesic
320. Meprobamate Component Of Equagesic
321. Meprobamate Component Of Micrainin
322. Ncgc00091031-02
323. Ncgc00257886-01
324. Mepro-aspirin Component Meprobamate
325. Smr000058750
326. Meprobamate Component Of Mepro-aspirin
327. 2-methyl-2-propyl-1,3-propanediyldicarbamate
328. D00376
329. 063m428
330. Q418351
331. Sr-01000937605
332. Sr-01000937605-2
333. W-105461
334. Meprobamate Solution, Drug Standard, 1.0 Mg/ml In Methanol
335. Meprobamate, European Pharmacopoeia (ep) Reference Standard
336. Meprobamate, United States Pharmacopeia (usp) Reference Standard
337. ({2-[(c-hydroxycarbonimidoyloxy)methyl]-2-methylpentyl}oxy)carboximidic Acid
338. Meprobamate Solution, 1.0 Mg/ml In Methanol, Ampule Of 1 Ml, Certified Reference Material
339. Diamino 3-methyl-3-propylglutarate; 2-methyl-2-propylpropane-1,3-diyl Dicarbamate; [2-(carbamoyloxymethyl)-2-methylpentyl] Carbamate
| Molecular Weight | 218.25 g/mol |
|---|---|
| Molecular Formula | C9H18N2O4 |
| XLogP3 | 0.7 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 8 |
| Exact Mass | 218.12665706 g/mol |
| Monoisotopic Mass | 218.12665706 g/mol |
| Topological Polar Surface Area | 105 Ų |
| Heavy Atom Count | 15 |
| Formal Charge | 0 |
| Complexity | 212 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Meprobamate |
| PubMed Health | Meprobamate (By mouth) |
| Drug Classes | Antianxiety |
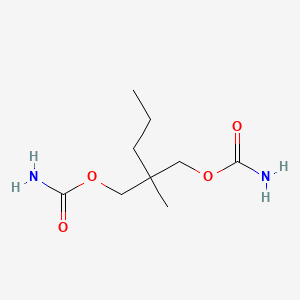
| Drug Label | Meprobamate is a white powder with a characteristic odor and a bitter taste. It is slightly soluble in water, freely soluble in acetone and alcohol, and sparingly soluble in ether. The structural formula of meprobamate is:C9H18N2O4... |
| Active Ingredient | Meprobamate |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 200mg; 400mg |
| Market Status | Prescription |
| Company | Alembic Pharms; Invagen Pharms; Watson Labs |
| 2 of 2 | |
|---|---|
| Drug Name | Meprobamate |
| PubMed Health | Meprobamate (By mouth) |
| Drug Classes | Antianxiety |
| Drug Label | Meprobamate is a white powder with a characteristic odor and a bitter taste. It is slightly soluble in water, freely soluble in acetone and alcohol, and sparingly soluble in ether. The structural formula of meprobamate is:C9H18N2O4... |
| Active Ingredient | Meprobamate |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 200mg; 400mg |
| Market Status | Prescription |
| Company | Alembic Pharms; Invagen Pharms; Watson Labs |
Anti-Anxiety Agents; Anticonvulsants; Muscle Relaxants, Central; Sedatives, Nonbarbiturate
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Medication (Vet): ... /Used occasionally/ as sedative with little or no tranquilization in many species.
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 337
Meprobamate has been used preoperatively to relieve anxiety and provide sedation. Meprobamate is effective in promoting sleep in the anxious, tense patient. There is no convincing evidence that meprobamate has any advantage over other sedatives, including barbiturates, in relieving anxiety and tension. /Included in US product label/
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009)
Meprobamate in fixed combination with aspirin is used as an adjunct in the short-term (<10 days) treatment of pain accompanied by tension and/or anxiety in patients with musculoskeletal disease. The combination has been shown to be more effective than aspirin alone. /Included in US product label/
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009)
Meprobamate is used for the management of anxiety disorders or for the short-term relief of the symptoms of anxiety. Anxiety or tension associated with the stress of everyday life usually does not require treatment with an anxiolytic. The efficacy of meprobamate for long-term use (i.e., longer than 4 months) has not been evaluated; the need for continued therapy should be reassessed periodically. Meprobamate may be useful as an adjunct when anxiety complicates psychosis or the treatment of alcohol dependence. However, the additive CNS depressant effects of meprobamate and alcohol should be considered. /Included in US product label/
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009)
Meprobamate frequently produces drowsiness. Other effects include nausea, vomiting, diarrhea, paresthesia, weakness, and CNS effects such as headache, paradoxical excitement, dizziness, ataxia, and disturbances of vision. There may be hypotensin, tachycardia, and cardiac dysrhythmias. Hypersensitivity reactions occur occasionally. These may be limited to skin rashes, urticaria, and purpura or may be more severe with angioedema, bronchospasm, or anuria. Erythema multiforme or Stevens-Johnson syndrome and exfoliative or bullous dermatitis have been reported. Blood disorders, including agranulocytosis, eosinophilia, leukopenia, thrombocytopenia, and aplastic anemia, have occasionally been reported.
Dart, R.C. (ed). Medical Toxicology. Third Edition, Lippincott Williams & Wilkins. Philadelphia, PA. 2004., p. 893-4
Clinical studies of meprobamate (with or without aspirin) did not include sufficient numbers of patients 65 years of age and older to determine whether geriatric patients respond differently than younger patients. While other clinical experience has not revealed age-related differences in response, drug dosage generally should be titrated carefully in geriatric patients, usually initiating therapy at the low end of the dosage range. The greater frequency of decreased hepatic, renal, and/or cardiac function and of concomitant disease and drug therapy observed in the elderly also should be considered.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009)
When meprobamate is used in fixed combination with aspirin, the usual cautions, precautions, and contraindications associated with aspirin must be considered in addition to those associated with meprobamate.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009)
Several studies have suggested and increased risk of congenital malformations associated with the use of anxiolytics (meprobamate, chlordiazepoxide, diazepam) during the first trimester of pregnancy. Since the use of anxiolytics is rarely urgent, their use during the first trimester should almost always be avoided. The possiblity that a woman of childbearing potential may be pregnant at the time therapy is initiated should be considered. Patients should be advised that if they become pregnant or intend to become pregnant during therapy, they should communicate with their physician about the desirability of discontinuing the drug.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009)
For more Drug Warnings (Complete) data for MEPROBAMATE (24 total), please visit the HSDB record page.
For the management of anxiety disorders or for the short-term relief of the symptoms of anxiety.
Meprobamate is an anxiolytic drug. It was the best selling minor tranquilizer for a time but has largely been replaced by benzodiazepines. Meprobamate has most of the pharmacological effects and dangers of the barbiturates (though it was marketed as being safer) but it is less sedating at effective doses. Meprobamate exhibits some anticonvulsant effects in absence seizures; however, it is reported to potentially exacerbate generalized tonic-clonic seizures. It has also been used as a hypnotic (sleeping pill). However, its is currently only licensed as an anxiolytic and it is a third or fourth-order choice.
Hypnotics and Sedatives
Drugs used to induce drowsiness or sleep or to reduce psychological excitement or anxiety. (See all compounds classified as Hypnotics and Sedatives.)
Anti-Anxiety Agents
Agents that alleviate ANXIETY, tension, and ANXIETY DISORDERS, promote sedation, and have a calming effect without affecting clarity of consciousness or neurologic conditions. ADRENERGIC BETA-ANTAGONISTS are commonly used in the symptomatic treatment of anxiety but are not included here. (See all compounds classified as Anti-Anxiety Agents.)
Anticonvulsants
Drugs used to prevent SEIZURES or reduce their severity. (See all compounds classified as Anticonvulsants.)
Muscle Relaxants, Central
A heterogeneous group of drugs used to produce muscle relaxation, excepting the neuromuscular blocking agents. They have their primary clinical and therapeutic uses in the treatment of muscle spasm and immobility associated with strains, sprains, and injuries of the back and, to a lesser degree, injuries to the neck. They have been used also for the treatment of a variety of clinical conditions that have in common only the presence of skeletal muscle hyperactivity, for example, the muscle spasms that can occur in MULTIPLE SCLEROSIS. (From Smith and Reynard, Textbook of Pharmacology, 1991, p358) (See all compounds classified as Muscle Relaxants, Central.)
N - Nervous system
N05 - Psycholeptics
N05B - Anxiolytics
N05BC - Carbamates
N05BC01 - Meprobamate
Absorption
Well absorbed from the gastrointestinal tract.
The concentrations of meprobamate in the blood and liver were determined in 12 cases of death due to meprobamate poisoning and in 29 cases of death due to the combined effects of meprobamate and alcohol, meprobamate and barbiturate, or meprobamate, alcohol and barbiturate. In the cases of poisoning from meprobamate alone, the blood meprobamate concentrations were within the range of 142-342 ug/mL. (mean value, 226 ug./mL). In the cases of poisoning with a combination of the drugs, the blood meprobamate concentrations were: for meprobamate and alcohol, 43-155 ug/mL (mean value, 117 ug/mL); for meprobamate and barbiturate, 30-276 ug/mL (mean value, 117 ug/mL); for meprobamate, barbiturate and alcohol, 33-460 ug/mL (mean value, 133 ug/mL).
Felby S; Acta Pharmacol. Toxicol 28 (5): 334-7 (1970)
Profound meprobamate intoxication is associated with plasma levels of 20 mg/100mL. Most pt appear to be comatose at levels about 10 mg/100 mL, whereas consciousness occurs at levels below 5 mg/100 mL.
Gosselin, R.E., R.P. Smith, H.C. Hodge. Clinical Toxicology of Commercial Products. 5th ed. Baltimore: Williams and Wilkins, 1984., p. III-260
The effect of hemoperfusion based on pharmacokinetic parameters in 4 cases of severe meprobamate intoxication is described. Maximal plasma levels reached 800, 816, 963 and 923 umol/L. All patients survived without sequelae including one patient resuscitated from cardiac arrest. The amount of meprobamate removed by hemoperfusion ranged from 1.6-6.2 g. Results suggest that hemoperfusion may be indicated in severe meprobamate intoxication.
PMID:3669117 Jacobsen D et al; J Toxicol Clin Toxicol 25 (4): 317-31 (1987)
Meprobamate is well absorbed from the GI tract following oral administration. Plasma concentrations of meprobamate required for sedative or hypnotic effects are not known. Oral administration of 400 mg of meprobamate produces peak plasma concentrations of 5-39 ug/mL within 1-3 hr. Plasma concentrations of 30-100 ug/mL are usually reached following mild overdosage and are associated with stupor or light coma. Plasma concentrations of 100-200 ug/mL are associated with deep coma and are potentially lethal; fatalities frequently occur when plasma concentrations exceed 200 ug/mL. The onset of sedative action is usually less than 1 hour following oral administration of meprobamate.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009)
For more Absorption, Distribution and Excretion (Complete) data for MEPROBAMATE (6 total), please visit the HSDB record page.
Meprobamate undergoes hepatic metabolism.
Meprobamate is rapidly metabolized in the liver. Meprobamate can induce liver microsomal enzymes and thus may at least theoretically alter the metabolism of other drugs such as warfarin. There is some evidence that meprobamate may accelerate its own metabolism. Meprobamate metabolites are inactive and include 2B-hydroxymeprobamate, and glucosyluronide and glucuronide conjugates of meprobamate. These metabolites are excreted by the kidneys. About 10-12% of a dose of meprobamate is excreted in urine as unchanged drug within 24 hr. The remainder is excreted in urine as metabolites.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009)
Most of the drug is metabolized in the liver, mainly to a side-chain hydroxy derivative and a glucuronide; the kinetics of elimination may depend on the dose.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 11th ed. New York, NY: McGraw-Hill, 2006., p. 422
A whole-body exposure of rats to 8 Gy radiation is ineffective in 3 days, and in 6 days, it prolongs considerably the effect and increases the pharmacological activity of hexenal, meprobamate, ethylmorphine, and amidopyrine, inhibits the activity of amidopyrine demethylase, aniline hydroxylase, NADPH-cytochrome c reductase, and reduces the content of protein, cytochromes P-450 and b5 in a microsomal liver fraction.
PMID:2780982 Khakimov ZZ; Radiobiologiia 29 (4): 492-4 (1989)
Carisoprodol is metabolized to meprobamate by the cytochrome P450 enzyme CYP2C19, encoded by the polymorphic CYP2C19 gene. Most studies on carisoprodol metabolism have been carried out on individuals phenotyped for CYP2C19 activity using the probe drug S-mephenytoin. /The investigators/ aimed to investigate whether the ratio of carisoprodol to meprobamate in a 'real life' setting could be predicted by CYP2C19 genotype or, more specifically, if high carisoprodol : meprobamate ratios in drugged drivers could be ascribed to the presence of mutant CYP2C19 alleles. From original material comprising 358 blood samples from apprehended drivers, two polarized groups were selected; a high-ratio group of 11 subjects where the carisoprodol : meprobamate ratio was >1 and a low-ratio control group of 23 subjects where the ratio was <0.31. Genotyping was carried out for the CYP2C19*2, CYP2C19*3 and CYP2C19*4 alleles. DNA samples from 94 healthy blood donors were used as reference material. The number of mutant alleles in the high-ratio and low-ratio groups was significantly higher and lower, respectively, than in the reference material. The increased number of mutant alleles in the high-ratio group was not due to the presence of many poor metabolizers, but to a high number of heterozygous individuals with the genotype CYP2C19*1/*2. This result indicates a gene dosage effect where the carisoprodol : meprobamate ratio reflects the number of active CYP2C19 alleles. The metabolism of carisoprodol to meprobamate is dependent on CYP2C19 genotype. Heterozygous individuals with the CYP2C19*1/*2 genotype have a reduced capacity for metabolizing carisoprodol, and should probably be regarded as intermediate metabolizers of this drug. /Carisoprodol/
PMID:12835613 Bramness JG et al; Pharmacogenetics 13 (7): 383-8 (2003).
Plasma half-life is about 10 hours.
The plasma half-life of meprobamate averages about 10-11 hours but may range from 6-16 hours.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009)
6-17 hours /From table/
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 11th ed. New York, NY: McGraw-Hill, 2006., p. 421
Meprobamate's mechanism of action is not fully understood; in animal studies, meprobamate is reported to act at multiple sites in the central nervous system, such as the thalamus and limbic system. It binds to the GABAA receptors, leading to inhibitory effects on the neurons transmitting signals in the reticular formation and spinal cord. Consequently, effects such as sedation and altered perception of pain are observed.
Meprobamate has CNS depressant actions similar to those of the barbiturates. The mechanism of action of meprobamate is not known. The drug apparently acts at multiple sites in the CNS including the hypothalamus, thalamus, limbic system, and spinal cord, but not the medulla, the reticular activating system, or in the autonomic nervous system. Although there is some evidence that meprobamate relaxes skeletal muscle tension, the skeletal muscle relaxant effects of the drug are probably caused by its sedative effect.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009)
The potentiation of the release of adenosine by meprobamate may account for its central effects.
Ullmann's Encyclopedia of Industrial Chemistry. 6th ed.Vol 1: Federal Republic of Germany: Wiley-VCH Verlag GmbH & Co. 2003 to Present, p. V30 433
In animals, meprobamate antagonizes the convulsant effects of pentylenetetrazol, but meprobamate is of no clinical use for the management of epilepsy; in fact, the drug may aggravate generalized tonic-clonic (grand mal) seizures. Usual sedative doses of meprobamate have little or no effect on the EEG, but 800-mg doses produce increased fast activity, increased amplitude, and prominent beta waves. Like barbiturates, hypnotic doses of meprobamate substantially suppress rapid eye movement (REM) or dreaming stage of sleep. REM rebound has been reported to occur when the drug was withdrawn and thus could cause the patient to experience markedly increased dreaming, nightmares, and/or insomnia.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009)

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
60
PharmaCompass offers a list of Meprobamate API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Meprobamate manufacturer or Meprobamate supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Meprobamate manufacturer or Meprobamate supplier.
PharmaCompass also assists you with knowing the Meprobamate API Price utilized in the formulation of products. Meprobamate API Price is not always fixed or binding as the Meprobamate Price is obtained through a variety of data sources. The Meprobamate Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Meprobamate manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Meprobamate, including repackagers and relabelers. The FDA regulates Meprobamate manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Meprobamate API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Meprobamate manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Meprobamate supplier is an individual or a company that provides Meprobamate active pharmaceutical ingredient (API) or Meprobamate finished formulations upon request. The Meprobamate suppliers may include Meprobamate API manufacturers, exporters, distributors and traders.
click here to find a list of Meprobamate suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Meprobamate DMF (Drug Master File) is a document detailing the whole manufacturing process of Meprobamate active pharmaceutical ingredient (API) in detail. Different forms of Meprobamate DMFs exist exist since differing nations have different regulations, such as Meprobamate USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Meprobamate DMF submitted to regulatory agencies in the US is known as a USDMF. Meprobamate USDMF includes data on Meprobamate's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Meprobamate USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Meprobamate suppliers with USDMF on PharmaCompass.
A Meprobamate CEP of the European Pharmacopoeia monograph is often referred to as a Meprobamate Certificate of Suitability (COS). The purpose of a Meprobamate CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Meprobamate EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Meprobamate to their clients by showing that a Meprobamate CEP has been issued for it. The manufacturer submits a Meprobamate CEP (COS) as part of the market authorization procedure, and it takes on the role of a Meprobamate CEP holder for the record. Additionally, the data presented in the Meprobamate CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Meprobamate DMF.
A Meprobamate CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Meprobamate CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Meprobamate suppliers with CEP (COS) on PharmaCompass.
A Meprobamate written confirmation (Meprobamate WC) is an official document issued by a regulatory agency to a Meprobamate manufacturer, verifying that the manufacturing facility of a Meprobamate active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Meprobamate APIs or Meprobamate finished pharmaceutical products to another nation, regulatory agencies frequently require a Meprobamate WC (written confirmation) as part of the regulatory process.
click here to find a list of Meprobamate suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Meprobamate as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Meprobamate API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Meprobamate as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Meprobamate and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Meprobamate NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Meprobamate suppliers with NDC on PharmaCompass.
Meprobamate Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Meprobamate GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Meprobamate GMP manufacturer or Meprobamate GMP API supplier for your needs.
A Meprobamate CoA (Certificate of Analysis) is a formal document that attests to Meprobamate's compliance with Meprobamate specifications and serves as a tool for batch-level quality control.
Meprobamate CoA mostly includes findings from lab analyses of a specific batch. For each Meprobamate CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Meprobamate may be tested according to a variety of international standards, such as European Pharmacopoeia (Meprobamate EP), Meprobamate JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Meprobamate USP).

