
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
FDA Orange Book
0
Canada
0
Australia
0
South Africa
0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
API
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
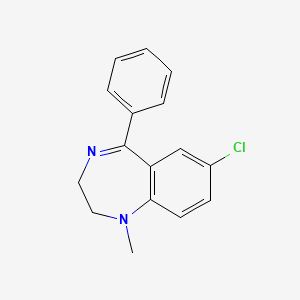

1. Hydrochloride, Medazepam
2. Medazepam Awd
3. Medazepam Hydrochloride
4. Medazepam Monohydrochloride
5. Monohydrochloride, Medazepam
6. Nobrium
7. Ro 5 4556
8. Ro 5-4556
9. Ro 54556
10. Rudotel
11. Rusedal
1. Nobrium
2. Rudotel
3. Ansilan
4. Resmit
5. 2898-12-6
6. Enobrin
7. Nivelton
8. Narsis
9. Pamnace
10. 1h-1,4-benzodiazepine, 7-chloro-2,3-dihydro-1-methyl-5-phenyl-
11. 7-chloro-2,3-dihydro-1-methyl-5-phenyl-1h-1,4-benzodiazepine
12. Chembl28333
13. 7-chloro-1-methyl-5-phenyl-2,3-dihydro-1,4-benzodiazepine
14. P0j3387w3s
15. Ansius
16. Nobrium Hcl
17. Ncgc00164523-01
18. 2,3-dihydro-7-chloro-1-methyl-5-phenyl-1h-1,4-benzodiazepine
19. 7-chloro-1-methyl-5-phenyl-2,3-dihydro-1h-1,4-benzodiazepine
20. 7-chloro-2,3-dihydro-1-methyl-5-phenyl-3h-1,4-benzodiazepine
21. 1h-1,4-benzodiazepine, 2,3-dihydro-7-chloro-1-methyl-5-phenyl-
22. Nobrium Hydrochloride
23. Aensius
24. Azepamide
25. Lerisum
26. Medaurin
27. Mezapam
28. Navizil
29. Nobraksin
30. Stratium
31. Benaon
32. Nobral
33. Nsc169896
34. Rb-252
35. Medazepamum [inn-latin]
36. Medazepam [inn:ban:jan]
37. Medazepamum
38. Psiquium
39. Dea No. 2836
40. Einecs 220-783-4
41. Pamnace (tn)
42. Diepin (salt/mix)
43. Elbrus (salt/mix)
44. Esmail (salt/mix)
45. Rb 252
46. Brn 0407983
47. Medazepol (salt/mix)
48. Megasedan (salt/mix)
49. Medazepam [inn]
50. Medazepam [jan]
51. Medazepam [mi]
52. Medazepam (jp17/inn)
53. Medazepam [mart.]
54. Medazepam [who-dd]
55. Dsstox_cid_28634
56. Dsstox_rid_82904
57. Dsstox_gsid_48708
58. Schembl18472
59. Unii-p0j3387w3s
60. Zinc1659
61. Dtxsid1048708
62. Chebi:31807
63. Tox21_113107
64. Bdbm50021058
65. Stl257370
66. Akos002254761
67. Db13437
68. Cas-2898-12-6
69. Db-047498
70. D01292
71. 5-23-09-00037 (beilstein Handbook Reference)
72. Q572796
73. 1-methyl-5-phenyl-7-chloro-2,3-dihydro-1h-1,4-benzodiazepine
74. 7-chloro-1-methyl-5-phenyl-2,3-dihydro-1h-[1,4]benzodiazepine
75. 7-chloro-1-methyl-5-phenyl-2,3-dihydro-1h-1,4-benzodiazepine #
76. 7-chloro-1-methyl-5-phenyl-2,3-dihydro-1h-benzo[e][1,4]diazepine
77. (z)-7-chloro-1-methyl-5-phenyl-2,3-dihydro-1h-benzo[e][1,4]diazepine
78. 1h-1,4-benzodiazepine, 7-chloro-2, 3-dihydro-1-methyl-5-phenyl, Monohydrochloride
79. 1h-1,4-benzodiazepine, 7-chloro-2,3-dihydro-1-methyl-5-phenyl-, Monohydrochloride
80. 7-chloro-2, 3-dihydro-1-methyl-5-phenyl-1h-1,4-benzodiazepine, Hydrochloride
81. 7-chloro-2,3-dihydro-1-methyl-5-phenyl-1h-1, 4-benzodiazepine Monohydrochloride
| Molecular Weight | 270.75 g/mol |
|---|---|
| Molecular Formula | C16H15ClN2 |
| XLogP3 | 4.4 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 1 |
| Exact Mass | 270.0923762 g/mol |
| Monoisotopic Mass | 270.0923762 g/mol |
| Topological Polar Surface Area | 15.6 Ų |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 336 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anticonvulsants
Drugs used to prevent SEIZURES or reduce their severity. (See all compounds classified as Anticonvulsants.)
Muscle Relaxants, Central
A heterogeneous group of drugs used to produce muscle relaxation, excepting the neuromuscular blocking agents. They have their primary clinical and therapeutic uses in the treatment of muscle spasm and immobility associated with strains, sprains, and injuries of the back and, to a lesser degree, injuries to the neck. They have been used also for the treatment of a variety of clinical conditions that have in common only the presence of skeletal muscle hyperactivity, for example, the muscle spasms that can occur in MULTIPLE SCLEROSIS. (From Smith and Reynard, Textbook of Pharmacology, 1991, p358) (See all compounds classified as Muscle Relaxants, Central.)
GABA Modulators
Substances that do not act as agonists or antagonists but do affect the GAMMA-AMINOBUTYRIC ACID receptor-ionophore complex. GABA-A receptors (RECEPTORS, GABA-A) appear to have at least three allosteric sites at which modulators act: a site at which BENZODIAZEPINES act by increasing the opening frequency of GAMMA-AMINOBUTYRIC ACID-activated chloride channels; a site at which BARBITURATES act to prolong the duration of channel opening; and a site at which some steroids may act. GENERAL ANESTHETICS probably act at least partly by potentiating GABAergic responses, but they are not included here. (See all compounds classified as GABA Modulators.)
Hypnotics and Sedatives
Drugs used to induce drowsiness or sleep or to reduce psychological excitement or anxiety. (See all compounds classified as Hypnotics and Sedatives.)
Anti-Anxiety Agents
Agents that alleviate ANXIETY, tension, and ANXIETY DISORDERS, promote sedation, and have a calming effect without affecting clarity of consciousness or neurologic conditions. ADRENERGIC BETA-ANTAGONISTS are commonly used in the symptomatic treatment of anxiety but are not included here. (See all compounds classified as Anti-Anxiety Agents.)
N - Nervous system
N05 - Psycholeptics
N05B - Anxiolytics
N05BA - Benzodiazepine derivatives
N05BA03 - Medazepam
Global Sales Information

Market Place

ABOUT THIS PAGE
21
PharmaCompass offers a list of Medazepam API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Medazepam manufacturer or Medazepam supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Medazepam manufacturer or Medazepam supplier.
PharmaCompass also assists you with knowing the Medazepam API Price utilized in the formulation of products. Medazepam API Price is not always fixed or binding as the Medazepam Price is obtained through a variety of data sources. The Medazepam Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Medazepam manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Medazepam, including repackagers and relabelers. The FDA regulates Medazepam manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Medazepam API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Medazepam manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Medazepam supplier is an individual or a company that provides Medazepam active pharmaceutical ingredient (API) or Medazepam finished formulations upon request. The Medazepam suppliers may include Medazepam API manufacturers, exporters, distributors and traders.
click here to find a list of Medazepam suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Medazepam Drug Master File in Japan (Medazepam JDMF) empowers Medazepam API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Medazepam JDMF during the approval evaluation for pharmaceutical products. At the time of Medazepam JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Medazepam suppliers with JDMF on PharmaCompass.
Medazepam Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Medazepam GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Medazepam GMP manufacturer or Medazepam GMP API supplier for your needs.
A Medazepam CoA (Certificate of Analysis) is a formal document that attests to Medazepam's compliance with Medazepam specifications and serves as a tool for batch-level quality control.
Medazepam CoA mostly includes findings from lab analyses of a specific batch. For each Medazepam CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Medazepam may be tested according to a variety of international standards, such as European Pharmacopoeia (Medazepam EP), Medazepam JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Medazepam USP).

