
Synopsis
Synopsis
0
CEP/COS
0
VMF
DRUG PRODUCT COMPOSITIONS
0
Weekly News Recap #Phispers

1. Act 064992
2. Act-064992
3. Act064992
4. Actelion-1
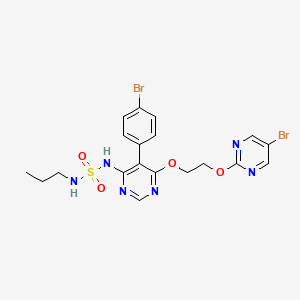
5. N-(5-(4-bromophenyl)-6-(2-(5-bromopyrimidin-2-yloxy)ethoxy)pyrimidin-4-yl)-n'-propylaminosulfonamide
6. Opsumit
1. 441798-33-0
2. Opsumit
3. Act-064992
4. Act 064992
5. N-[5-(4-bromophenyl)-6-[2-[(5-bromo-2-pyrimidinyl)oxy]ethoxy]-4-pyrimidinyl]-n'-propylsulfamide
6. Act064992
7. Z9k9y9wmvl
8. 5-(4-bromophenyl)-6-[2-(5-bromopyrimidin-2-yl)oxyethoxy]-n-(propylsulfamoyl)pyrimidin-4-amine
9. N-(5-(4-bromophenyl)-6-(2-((5-bromo-2-pyrimidinyl)oxy)ethoxy)-4-pyrimidinyl)-n'-propylsulfamide
10. Chebi:76607
11. Actelion-1
12. Macitentan [inn]
13. N-(5-(4-bromophenyl)-6-(2-((5-bromopyrimidin-2-yl)oxy)ethoxy)pyrimidin-4-yl)-n'-propylsulfamide
14. N-[5-(4-bromophenyl)-6-{2-[(5-bromopyrimidin-2-yl)oxy]ethoxy}pyrimidin-4-yl]-n'- Propylsulfamide
15. Macitentan [usan:inn]
16. Unii-z9k9y9wmvl
17. Macitentanum
18. Sulfamide, N-(5-(4-bromophenyl)-6-(2-((5-bromo-2-pyrimidinyl)oxy)ethoxy)-4-pyrimidinyl)-n'-propyl-
19. Sulfamide, N-[5-(4-bromophenyl)-6-[2-[(5-bromo-2-pyrimidinyl)oxy]ethoxy]-4-pyrimidinyl]-n'-propyl-
20. Macitentan- Bio-x
21. Opsumit (tn)
22. Macitentan [mi]
23. Macitentan [jan]
24. Macitentan (jan/usan)
25. Macitentan [usan]
26. Macitentan [vandf]
27. (non-labelled)macitentan-d7
28. Macitentan [who-dd]
29. Mls006011174
30. Gtpl7352
31. Schembl1445625
32. Chembl2103873
33. Macitentan [orange Book]
34. Dtxsid50196063
35. Ex-a544
36. Hms3653n06
37. Hms3747e09
38. Cas:441798-33-0;macitentan
39. Bcp05309
40. Bdbm50395626
41. Mfcd17167076
42. S8051
43. Zinc43202140
44. Akos024463406
45. Am81244
46. Ccg-270155
47. Cs-0686
48. Db08932
49. Sb14841
50. Macitentan (actelion-1,act-064992)
51. Ncgc00346456-01
52. Ncgc00346456-05
53. Ac-30102
54. As-74590
55. Bm162771
56. Hy-14184
57. N-(5-(4-bromophenyl)-6-(2-(5-bromopyrimidin-2-yloxy)ethoxy)pyrimidin-4-yl)-n'-propylaminosulfonamide
58. Smr004702943
59. Db-070519
60. Ft-0696675
61. Sw219473-1
62. Act 064992; Act-064992
63. D10135
64. Q6724151
65. {[5-(4-bromophenyl)-6-{2-[(5-bromopyrimidin-2-yl)oxy]ethoxy}pyrimidin-4-yl]sulfamoyl}(propyl)amine
66. N-(5-(4-bromophenyl)-6-(2-((5-bromopyrimidin-2-yl)oxi)ethoxy)pyrimidin-4-yl)-n'-propylsulfuric Diamide
67. N-[5-(4-bromophenyl)-6-[2-[(5-bromo-2-pyrimidinyl)-oxy]ethoxy]-4-pyrimidinyl]-n'-propylsulfamide
68. N-[5-(4-bromophenyl)-6-[2-[(5-bromo-2-pyrimidinyl)oxy]ethoxy]-4-pyrimidinyl]-n'-propyl-sulfamide
69. N-[5-(4-bromophenyl)-6-{2-[(5-bromopyrimidin-2-yl)oxy]ethoxy}pyrimidin-4-yl]-n'-propylsulfuric Diamide
| Molecular Weight | 588.3 g/mol |
|---|---|
| Molecular Formula | C19H20Br2N6O4S |
| XLogP3 | 3.7 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 11 |
| Exact Mass | 587.96130 g/mol |
| Monoisotopic Mass | 585.96335 g/mol |
| Topological Polar Surface Area | 137 Ų |
| Heavy Atom Count | 32 |
| Formal Charge | 0 |
| Complexity | 642 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Opsumit |
| PubMed Health | Macitentan (By mouth) |
| Drug Classes | Antihypertensive |
| Drug Label | OPSUMIT (macitentan) is an endothelin receptor antagonist. The chemical name of macitentan is N-[5-(4-Bromophenyl)-6-[2-[(5-bromo-2-pyrimidinyl)oxy]ethoxy]-4-pyrimidinyl]-N'-propylsulfamide. It has a molecular formula of C19H20Br2N6O4S and a molecula |
| Active Ingredient | Macitentan |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 10mg |
| Market Status | Prescription |
| Company | Actelion Pharms |
| 2 of 2 | |
|---|---|
| Drug Name | Opsumit |
| PubMed Health | Macitentan (By mouth) |
| Drug Classes | Antihypertensive |
| Drug Label | OPSUMIT (macitentan) is an endothelin receptor antagonist. The chemical name of macitentan is N-[5-(4-Bromophenyl)-6-[2-[(5-bromo-2-pyrimidinyl)oxy]ethoxy]-4-pyrimidinyl]-N'-propylsulfamide. It has a molecular formula of C19H20Br2N6O4S and a molecula |
| Active Ingredient | Macitentan |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 10mg |
| Market Status | Prescription |
| Company | Actelion Pharms |
Investigated for use/treatment in cardiovascular disorders, hypertension, and pulmonary hypertension.
Macitentan is indicated for the treatment of WHO group 1 pulmonary arterial hypertension (PAH) both alone and in combination with tadalafil.
FDA Label
Opsumit, as monotherapy or in combination, is indicated for the long-term treatment of pulmonary arterial hypertension (PAH) in adult patients of WHO Functional Class (FC) II to III.
Efficacy has been shown in a PAH population including idiopathic and heritable PAH, PAH associated with connective tissue disorders, and PAH associated with corrected simple congenital heart disease.
Treatment of chronic thromboembolic pulmonary hypertension (CTEPH)
Treatment of idiopathic pulmonary fibrosis, Treatment of pulmonary arterial hypertension, Treatment of systemic sclerosis
Treatment of functional single ventricle heart disease with total cavo-pulmonary connection
Macitentan acts primarily by reducing vasoconstriction and cell proliferation due to endothelin overexpression.
Endothelin A Receptor Antagonists
Compounds and drugs that bind to and inhibit or block the activation of ENDOTHELIN A RECECPTORS. (See all compounds classified as Endothelin A Receptor Antagonists.)
Endothelin B Receptor Antagonists
Compounds and drugs that bind to and inhibit or block the activation of ENDOTHELIN B RECEPTORS. (See all compounds classified as Endothelin B Receptor Antagonists.)
C02KX04
C02KX04
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
C - Cardiovascular system
C02 - Antihypertensives
C02K - Other antihypertensives
C02KX - Antihypertensives for pulmonary arterial hypertension
C02KX04 - Macitentan
Absorption
Macitentan has a median Tmax of 8h although some studies have found up to 30h at higher doses. Although the bioavailability has not been experimentally determined, pharmacokinetic modeling has estimated it at 74%. Food has not been found to have a significant effect on absorption.
Route of Elimination
Eliminated 50% through urine and 24% through feces. Of the 50% excreted through the urine, none of the recovered dose was in the form of the parent drug nor the active metabolite.
Volume of Distribution
Macitentan has an apparent volume of distribution of 40-50L.
Clearance
Clearance data was not found.
Macitentan undergoes oxidative depropylation of the sulfonamide moiety via CYP3A4, 2C8, 2C9, and 2C19 to form the active metabolite M6. The ethylene glycol moiety undergoes oxidative cleavage via CYP2C9 to the alcohol metabolite M4. M4 is oxidized to its corresponding acid, M5, then hydrolyzed to the metabolite termed m/z 324. Oxidative depropylation of a distal carbon atom via CYP2C8, 2C9, and 2C19 forms M7. Hydrolysis of both macitentan and M5 produces M3. Finally M5 may be further metabolized via hydrolysis and hydroxylation to M2 or via glucuronidation to a glucuronide metabolite, M1.
The half-life of elimination of macitentan is 16 hours. The half-life of elimination of the active metabolite is 40-66h
Through complete blockade of tissular endothelin, Actelion-1 is expected to protect tissue from the damaging effect of elevated endothelin, specifically in the cardiovascular system. In pre-clinical studies, Actelion-1 also exhibited effects suggesting that it maintains the integrity of the vascular wall and improves long-term outcome. Accordingly, Actelion-1 may provide therapeutic benefit in a wide range of cardiovascular indications.
Macitentan is an antagonist which binds to the endothelin A and B receptors (EA and EB) and blocks signaling from endothelin-1 and -2. Pulmonary arterial hypertension has many different mechanisms which contribute to the development of endothelial dysfunction including elevated cytosolic calcium, genetic factors, epigenetic changes, and mitochondrial dysfunction. The focus of macitentan's mechanism relates to the role of overexpressed endothelin from the vascular endothelium. Endothelins are released in both a constitutive fashion from secretory vesicles and in response to stimuli via Weibel-Palade storage granules. Endothelins bind to the EA and EB receptors, with endothelins -1 and -2 having more affinity than endothelin-3. Binding to the Gq coupled EA receptor triggers Ca2+ release from the sarcoplasmic reticulum of smooth muscle cells via the phospholipase C (PLC) pathway. Downstream protein kinase C activation may also contribute to increased Ca2+ sensitivity of the contractile apparatus. EA receptor activation is also known to contribute to pulmonary artery smooth muscle cell proliferation. The binding of endothelins to the EB receptors acts in opposition to EA signaling by activating the same PLC cascade in endothelial cells to activate endothelial nitric oxide synthase. The subsequent release of nitric oxide produces vasodilation through the cyclic guanosine monophosphate cascade. Despite the greater presence of EB receptors on endothelial cells, they are still present on smooth muscle cells and may contribute to cell proliferation through the same mechanisms as EA receptors. Macitentan is thought to provide its therapeutic effect primarily via blocking signaling through EA which produces both decreased vasoconstriction via reduced smooth muscle cell contractility and attenuation of the hyperproliferation of smooth muscle cells found in PAH. Blockade of EB is less likely to contribute to a therapeutic effect as this signaling is responsible for the counter-regulatory vasodilatory signal.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
5-(4-BROMOPHENYL)-4,6-DICHLOROPYRIMIDINE
CAS Number : 146533-41-7
End Use API : Macitentan
About The Company : Founded with a mission to transform strategic capital into specialty chemicals, Ami Group focuses on Agrochemicals, Cosmetics, and Polymers. Ami Organics Ltd. i...

5-(4-bromophenyl)-4,6-dichloropyrimidine
CAS Number : 146533-41-7
End Use API : Macitentan
About The Company : Beijing Cooperate Pharmaceutical Co.,Ltd is a high-tech company specializing in pharmaceutical and advanced material, involving API,pharmaceutical intermediates...

2-(4-Bromophenyl)-propanedioic acid, 1,3-mdiethyl ...
CAS Number : 149506-35-4
End Use API : Macitentan
About The Company : Beijing Cooperate Pharmaceutical Co.,Ltd is a high-tech company specializing in pharmaceutical and advanced material, involving API,pharmaceutical intermediates...

5-(4-Bromophenyl)pyrimidine-4,6-diol
CAS Number : 706811-25-8
End Use API : Macitentan
About The Company : Beijing Cooperate Pharmaceutical Co.,Ltd is a high-tech company specializing in pharmaceutical and advanced material, involving API,pharmaceutical intermediates...

CAS Number : 147962-41-2
End Use API : Macitentan
About The Company : Beijing Cooperate Pharmaceutical Co.,Ltd is a high-tech company specializing in pharmaceutical and advanced material, involving API,pharmaceutical intermediates...

CAS Number : 147962-41-2
End Use API : Macitentan
About The Company : Our journey began in 1969, with our first manufacturing facility Jet Chemicals Pvt. Ltd (JCPL), a pioneer in manufacturing Pharmaceutical Excipients (Saccharin ...

CAS Number : 147962-41-2
End Use API : Macitentan
About The Company : Blue Jet Healthcare is a global, science-driven pharmaceutical company specializing in collaboration, development, and manufacturing of advanced pharmaceutical ...

5-(4-Bromophenyl)-4,6-dichloropyrimidine
CAS Number : 146533-41-7
End Use API : Macitentan
About The Company : Glenfin Chemicals, a trusted provider of high-quality chemical solutions tailored to meet the diverse needs of industries worldwide. Based in the heart of inno...

5-4-bromophenyl-4,6-dichloropyrimidine
CAS Number : 146533-41-7
End Use API : Macitentan
About The Company : Established in 2011 and situated in Hangzhou, Zhejiang, China, Hangzhou Longshine Bio-Tech CO., Ltd is dedicated to providing services for pharmaceutical and ch...

N -propylsulfamide
CAS Number : 147962-41-2
End Use API : Macitentan
About The Company : Established in 2011 and situated in Hangzhou, Zhejiang, China, Hangzhou Longshine Bio-Tech CO., Ltd is dedicated to providing services for pharmaceutical and ch...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Related Excipient Companies

Dosage Form : Tablet
Grade : Not Available
Brand Name : Kollicoat Protect
Application : Coating Systems & Additives
Excipient Details : Flexible water soluble instant release coating polymer, especially used for moisture protection
Pharmacopoeia Ref : Excipient based on Kollicoat®...
Technical Specs : Not Available
Ingredient(s) : Polyvinyl Alcohol Graft Polyethylene Glycol Copolymer
Dosage Form : Capsule
Grade : Not Available
Application : Solubilizers
Excipient Details : Solubilizing matrix, crystallization inhibitor & stabilizer in injectables and ophthalmic products
Dosage Form : Capsule
Grade : Not Available
Application : Solubilizers
Excipient Details : Solubilizing matrix, crystallization inhibitor & stabilizer in injectables and ophthalmic products
Pharmacopoeia Ref : Ph. Eur., USP: Povidone; JPE: ...
Technical Specs : Not Available
Ingredient(s) : Povidone
Dosage Form : Tablet
Grade : Not Available
Application : Controlled & Modified Release
Excipient Details : Solubilization, dispersion, crystallization inhibition, instant release matrices & spray drying
Dosage Form : Tablet
Grade : Not Available
Application : Solubilizers
Excipient Details : Solubilization, dispersion, crystallization inhibition, instant release matrices & spray drying
Pharmacopoeia Ref : Ph. Eur., USP, JP: Povidone
Technical Specs : Not Available
Ingredient(s) : Povidone
Dosage Form : Tablet
Grade : Not Available
Application : Fillers, Diluents & Binders
Excipient Details : Binder for peroxide sensitive drugs in solid oral dosage forms. Drug solubilizer with low peroxide in transdermal patches.
Pharmacopoeia Ref : Ph. Eur., USP, JP: Povidone
Technical Specs : Not Available
Ingredient(s) : Povidone
Excipients Web Link
Dosage Form : Capsule
Grade : Not Available
Application : Fillers, Diluents & Binders
Excipient Details : Tablet binding, thickener, stabilizers of suspensions, reduces sedimentation, crystallization inhibition.
Pharmacopoeia Ref : Ph. Eur., USP, JP: Povidone
Technical Specs : Not Available
Ingredient(s) : Povidone
Dosage Form : Tablet
Grade : Not Available
Application : Granulation
Excipient Details : For non-erodible matrices using direct compression, Controlled release matrix. Matrix former in transdermal patches and topical films.
Pharmacopoeia Ref : Ph. Eur., USP-NF, JP-JPE: 80 %...
Technical Specs : Not Available
Ingredient(s) : Povidone
Dosage Form : Softgel Capsule
Grade : Not Available
Application : Solubilizers
Excipient Details : Nonionic solubilizer, emulsifier and co-emulsifier
Dosage Form : Tablet
Grade : Not Available
Application : Direct Compression
Excipient Details : Ready-to-use direct compression solution for tablets.
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Dosage Form : Tablet
Grade : Oral
Application : Taste Masking
Excipient Details : F-Melt Type C is a pharmaceutical excipient used in oral dosage forms like orally disintegrating tablets, sachets, dispersible tablets, chewable tablets and sublingual tablets.
Pharmacopoeia Ref : Conforms to Japanese Pharmaceu...
Technical Specs : Not Available
Ingredient(s) : Crospovidone
Dosage Form : Orodispersible Tablet
Grade : Oral
Application : Disintegrants & Superdisintegrants
Excipient Details : F-Melt Type M is used in various dosage forms like orally disintegrating tablets (ODTs), sachets, dispersible tablets, chewable tablets and sublingual formulations.
Pharmacopoeia Ref : Conforms to Japanese Pharmaceu...
Technical Specs : Not Available
Ingredient(s) : Crospovidone
Dosage Form : Ophthalmic Solution
Grade : Oral, Ophthalmic, Microsphere Injectable,Topical
Application : Parenteral
Excipient Details : Gohsenol EG acts as a pharmaceutical binder, filler and film former in various dosage forms like opthalmic, microsphere, OD strip and gel patches.
Pharmacopoeia Ref : JPE, USP, EP, ChP (China)
Technical Specs : Highly purified PVA, Partially hydrolysis, having all viscocity grade from 3cps to 48 cps.
Ingredient(s) : Polyvinyl Alcohol
Dosage Form : Capsule
Grade : Oral
Application : Fillers, Diluents & Binders
Pharmacopoeia Ref : USP-NF, EP, BP, IP, JP, FCC
Technical Specs : PVP K-K-30/ K-17/ K19/ K25/ K90
Ingredient(s) : Povidone
Dosage Form : Tablet
Grade : Oral, Topical
Brand Name : MONTANOX 80 PHA PREMIUM
Application : Emulsifying Agents
Pharmacopoeia Ref : USP-NF, EP, JP
Technical Specs : Non-Ionic Hydrophilic surfactant, Emulsifier (o/w emulsion), Solubilizer
Ingredient(s) : Polysorbate 80
Dosage Form : Capsule
Grade : Oral
Application : Coating Systems & Additives
Excipient Details : Ready mix Film coating system for moisture sensitive APIs
Pharmacopoeia Ref : USP, EP, JP; Having US-DMF
Technical Specs : Moisture barrier film coating system
Ingredient(s) : Hydroxypropyl Methyl Cellulose
Dosage Form : Capsule
Grade : Oral
Application : Coating Systems & Additives
Excipient Details : Ready mix Non-Functional film coating system.
Pharmacopoeia Ref : USP, EP, JP; Having US-DMF
Technical Specs : Not Available
Ingredient(s) : Hydroxypropyl Methyl Cellulose
Dosage Form : Capsule
Grade : Oral
Application : Taste Masking
Excipient Details : Ready mix sugar coating system.
Pharmacopoeia Ref : USP, EP, JP & having US DMF
Technical Specs : Sprayable sugar coating system for solid oral dosage form
Ingredient(s) : Hydroxypropyl Methyl Cellulose
Dosage Form : Emulsion
Grade : Topical
Application : Rheology Modifiers
Excipient Details : Thickener, Emulsifier, Stabilizer, Texturizing agent, pH Independent & Non Thixotropic polymer for Topical Range (Skin,Vaginal & Anal mucosa)
Pharmacopoeia Ref : In house having US DMF Type IV...
Technical Specs : Ready to use liquid polymer for topical applications (Gel / Cream / Lotion/ Foam based formulation, ...
Ingredient(s) : Hydroxyethyl Acrylate
Dosage Form : Capsule
Grade : Oral
Application : Solubilizers
Excipient Details : Polysorbate 80 in dry powder form, a solubilizing agent acts as a surfactant and increases the solubility of various oral dosage forms.
Pharmacopoeia Ref : USP-NF, EP, JP & having US DMF
Technical Specs : Solubilizer in powder form, used in directly compressible dosage forms, Wet granulation, added durin...
Ingredient(s) : Magnesium aluminium silicate Excipient
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Dosage Form : Tablet
Grade : Oral
Application : Controlled & Modified Release
Excipient Details : Espheres EM can be used as an inert base for modified release formulations promoting uniformity of release profile.
Pharmacopoeia Ref : Not Available
Technical Specs : Not Available
Ingredient(s) : Microcrystalline Cellulose Excipients
Dosage Form : Tablet
Grade : Oral
Application : Coating Systems & Additives
Excipient Details : Instacoat EMB is an excellent moisture barrier which reduces degradation of moisture sensitive APIs.
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Dosage Form : Tablet
Grade : Oral
Application : Fillers, Diluents & Binders
Excipient Details : TABCELL serves as an excellent excipient for solid dosage forms, providing numerous advantages for tablet formulations.
Pharmacopoeia Ref : Not Available
Technical Specs : Not Available
Ingredient(s) : Microcrystalline Cellulose Excipients
Dosage Form : Tablet
Grade : Oral
Application : Lubricants & Glidants
Excipient Details : TABLUBE is one of the oldest and most widely used lubricants for tablet, capsules and other solid dosage forms.
Pharmacopoeia Ref : Not Available
Technical Specs : Not Available
Ingredient(s) : Magnesium Stearate
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
65
PharmaCompass offers a list of Macitentan API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Macitentan manufacturer or Macitentan supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Macitentan manufacturer or Macitentan supplier.
PharmaCompass also assists you with knowing the Macitentan API Price utilized in the formulation of products. Macitentan API Price is not always fixed or binding as the Macitentan Price is obtained through a variety of data sources. The Macitentan Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Macitentan manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Macitentan, including repackagers and relabelers. The FDA regulates Macitentan manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Macitentan API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Macitentan manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Macitentan supplier is an individual or a company that provides Macitentan active pharmaceutical ingredient (API) or Macitentan finished formulations upon request. The Macitentan suppliers may include Macitentan API manufacturers, exporters, distributors and traders.
click here to find a list of Macitentan suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Macitentan DMF (Drug Master File) is a document detailing the whole manufacturing process of Macitentan active pharmaceutical ingredient (API) in detail. Different forms of Macitentan DMFs exist exist since differing nations have different regulations, such as Macitentan USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Macitentan DMF submitted to regulatory agencies in the US is known as a USDMF. Macitentan USDMF includes data on Macitentan's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Macitentan USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Macitentan suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Macitentan Drug Master File in Japan (Macitentan JDMF) empowers Macitentan API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Macitentan JDMF during the approval evaluation for pharmaceutical products. At the time of Macitentan JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Macitentan suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Macitentan Drug Master File in Korea (Macitentan KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Macitentan. The MFDS reviews the Macitentan KDMF as part of the drug registration process and uses the information provided in the Macitentan KDMF to evaluate the safety and efficacy of the drug.
After submitting a Macitentan KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Macitentan API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Macitentan suppliers with KDMF on PharmaCompass.
A Macitentan written confirmation (Macitentan WC) is an official document issued by a regulatory agency to a Macitentan manufacturer, verifying that the manufacturing facility of a Macitentan active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Macitentan APIs or Macitentan finished pharmaceutical products to another nation, regulatory agencies frequently require a Macitentan WC (written confirmation) as part of the regulatory process.
click here to find a list of Macitentan suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Macitentan as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Macitentan API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Macitentan as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Macitentan and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Macitentan NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Macitentan suppliers with NDC on PharmaCompass.
Macitentan Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Macitentan GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Macitentan GMP manufacturer or Macitentan GMP API supplier for your needs.
A Macitentan CoA (Certificate of Analysis) is a formal document that attests to Macitentan's compliance with Macitentan specifications and serves as a tool for batch-level quality control.
Macitentan CoA mostly includes findings from lab analyses of a specific batch. For each Macitentan CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Macitentan may be tested according to a variety of international standards, such as European Pharmacopoeia (Macitentan EP), Macitentan JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Macitentan USP).

