



API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
0
Listed Suppliers
0
0
0

USA (Orange Book)

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
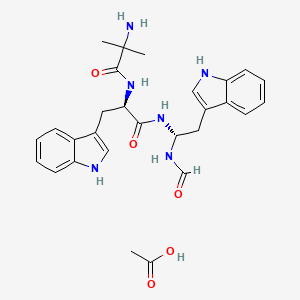

1. Aezs-130
2. Aib-dtrp-dgtrp-cho
3. Aib-trp-gtrp-cho
4. Aminoisobutyryl-tryptophyl-tryptophanamine-formyl
5. Ard-07
6. Ep 1572
7. Ep-1572
8. Ep1572
9. Jmv 1843
10. Jmv-1843
11. Jmv1843
12. Macimorelin
13. Macrilen
1. Macrilen
2. D-87575 Acetate
3. Macimorelin Acetate [usan]
4. 945212-59-9
5. Aqz1003rmg
6. Macimorelin Acetate (usan)
7. D-tryptophanamide, 2-methylalanyl-n-((1r)-1-(formylamino)-2-(1h-indol-3-yl)ethyl)-, Acetate (1:1)
8. N2-(2-amino-2-methylpropanoyl-n1-((1r)-1-formamido-2-(1h-indol-3-yl)ethyl)- D-tryptophanamide Acetate
9. Ard 07 Acetate
10. Ep 1572 Acetate
11. Unii-aqz1003rmg
12. Aezs 130
13. Macrilen (tn)
14. Ard-07 Acetate
15. Aib-trp-gtrp-cho Acetate
16. Ep-1572 Acetate
17. Chembl2364617
18. Macimorelin Acetate [mi]
19. Dtxsid40241516
20. Macimorelin Acetate [who-dd]
21. Macimorelin Acetate [orange Book]
22. D10562
23. Q27274071
| Molecular Weight | 534.6 g/mol |
|---|---|
| Molecular Formula | C28H34N6O5 |
| Hydrogen Bond Donor Count | 7 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 9 |
| Exact Mass | 534.25906821 g/mol |
| Monoisotopic Mass | 534.25906821 g/mol |
| Topological Polar Surface Area | 182 Ų |
| Heavy Atom Count | 39 |
| Formal Charge | 0 |
| Complexity | 792 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
This medicinal product is for diagnostic use only. GHRYVELIN is indicated for the diagnosis of growth hormone deficiency (GHD) in adults.
V04CD06


