
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
VMF
0
Australia
Annual Reports
NA
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
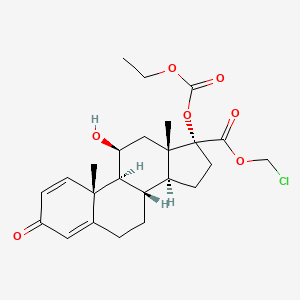

1. 17-ethoxycarbonyloxy-11-hydroxy-3-oxoandrosta-1,4-diene-17-carboxylate, Chloromethyl
2. Alrex
3. Cehoac
4. Chloromethyl 17 Ethoxycarbonyloxy 11 Hydroxy 3 Oxoandrosta 1,4 Diene 17 Carboxylate
5. Chloromethyl 17-ethoxycarbonyloxy-11-hydroxy-3-oxoandrosta-1,4-diene-17-carboxylate
6. Etabonate, Loteprednol
7. Lotemax
8. Loteprednol
1. 82034-46-6
2. Lotemax
3. Alrex
4. Hgp-1
5. Inveltys
6. Cddd-5604
7. P-5604
8. Cddd 5604
9. Chloromethyl (8s,9s,10r,11s,13s,14s,17r)-17-ethoxycarbonyloxy-11-hydroxy-10,13-dimethyl-3-oxo-7,8,9,11,12,14,15,16-octahydro-6h-cyclopenta[a]phenanthrene-17-carboxylate
10. Chebi:31784
11. Yeh1ez96k6
12. Kpi-121
13. Dsstox_cid_26468
14. Dsstox_rid_81641
15. Dsstox_gsid_46468
16. (11b,17a)-17-[(ethoxycarbonyl)oxy]-11-hydroxy-3-oxo-androsta-1,4-diene-17-carboxylic Acid Chloromethyl Ester
17. Chloromethyl 17alpha-[(ethoxycarbonyl)oxy]-11beta-hydroxy-3-oxoandrosta-1,4-diene-17beta-carboxylate
18. Loterox
19. Locort
20. Lotemax (tn)
21. Cas-82034-46-6
22. Unii-yeh1ez96k6
23. Hgp 1
24. Loteprednol Etabonate [usan]
25. Airex
26. Loteprednol Etabonate (jan/usan)
27. Loteprednoletabonate
28. Ncgc00164594-01
29. Idr-90102
30. Idr-90103
31. Eysuvis
32. Loteprednol Etabonate Opphthalmic Suspension
33. P 5604
34. Schembl23907
35. Mls001424221
36. Gtpl7085
37. Chembl1200865
38. Dtxsid2046468
39. Hms2051f16
40. Hms2232j09
41. Hms3715n06
42. Loteprednol Etabonate [mi]
43. Loteprednol Etabonate [usan:jan]
44. Loteprednol Etabonate [jan]
45. Bcp28645
46. Zinc3920673
47. Tox21_112219
48. Bdbm50248301
49. Loteprednol Etabonate [vandf]
50. Mfcd00870765
51. S1669
52. Loteprednol Etabonate [mart.]
53. Akos005145741
54. Loteprednol Etabonate [who-dd]
55. Tox21_112219_1
56. Ccg-101041
57. Cs-0900
58. Db14596
59. Gs-3599
60. Nc00291
61. Loteprednol Etabonate, >=98% (hplc)
62. Ncgc00164594-02
63. Chloromethyl 11beta,17-dihydroxy-3-oxoandrosta-1,4-diene-17beta-carboxylate, 17-(ethyl Carbonate)
64. Hy-17358
65. Smr000469178
66. Loteprednol Etabonate [orange Book]
67. Zylet Component Loteprednol Etabonate
68. L0327
69. D01689
70. Loteprednol Etabonate Component Of Zylet
71. Ab00698349-05
72. Ab00698349-07
73. Ab00698349_08
74. 034l466
75. Q3837481
76. Androsta-1,4-diene-17-carboxylic Acid, 17-((ethoxycarbonyl)oxy)-11-hydroxy-3-oxo-, Chloromethyl Ester, (11.beta.,17.alpha.)-
77. Androsta-1,4-diene-17-carboxylic Acid, 17-((ethoxycarbonyl)oxy)-11-hydroxy-3-oxo-, Chloromethyl Ester, (11beta,17alpha)-
78. Chloromethyl (1s,2r,10s,11s,14r,15s,17s)-14-[(ethoxycarbonyl)oxy]-17-hydroxy-2,15-dimethyl-5-oxotetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadeca-3,6-diene-14-carboxylate
79. Chloromethyl 11.beta.,17-dihydroxy-3-oxoandrosta-1,4-diene-17.beta.-carboxylate, 17-(ethyl Carbonate)
| Molecular Weight | 466.9 g/mol |
|---|---|
| Molecular Formula | C24H31ClO7 |
| XLogP3 | 3.9 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 7 |
| Exact Mass | 466.1758310 g/mol |
| Monoisotopic Mass | 466.1758310 g/mol |
| Topological Polar Surface Area | 99.1 Ų |
| Heavy Atom Count | 32 |
| Formal Charge | 0 |
| Complexity | 882 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 7 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Alrex |
| Drug Label | ALREX (loteprednol etabonate ophthalmic suspension) contains a sterile, topical anti-inflammatory corticosteroid for ophthalmic use.Loteprednol etabonate is a white to off-white powder.Loteprednol etabonate is represented by the following structura... |
| Active Ingredient | Loteprednol etabonate |
| Dosage Form | Suspension/drops |
| Route | Ophthalmic |
| Strength | 0.2% |
| Market Status | Prescription |
| Company | Bausch And Lomb |
| 2 of 4 | |
|---|---|
| Drug Name | Lotemax |
| PubMed Health | Loteprednol (Into the eye) |
| Drug Classes | Ophthalmologic Agent |
| Drug Label | LOTEMAX (loteprednol etabonate ophthalmic suspension) contains a sterile, topical anti-inflammatory corticosteroid for ophthalmic use. Loteprednol etabonate is a white to off-white powder.Loteprednol etabonate is represented by the following struct... |
| Active Ingredient | Loteprednol etabonate |
| Dosage Form | Ointment; Gel; Suspension/drops |
| Route | Ophthalmic |
| Strength | 0.5% |
| Market Status | Prescription |
| Company | Bausch And Lomb |
| 3 of 4 | |
|---|---|
| Drug Name | Alrex |
| Drug Label | ALREX (loteprednol etabonate ophthalmic suspension) contains a sterile, topical anti-inflammatory corticosteroid for ophthalmic use.Loteprednol etabonate is a white to off-white powder.Loteprednol etabonate is represented by the following structura... |
| Active Ingredient | Loteprednol etabonate |
| Dosage Form | Suspension/drops |
| Route | Ophthalmic |
| Strength | 0.2% |
| Market Status | Prescription |
| Company | Bausch And Lomb |
| 4 of 4 | |
|---|---|
| Drug Name | Lotemax |
| PubMed Health | Loteprednol (Into the eye) |
| Drug Classes | Ophthalmologic Agent |
| Drug Label | LOTEMAX (loteprednol etabonate ophthalmic suspension) contains a sterile, topical anti-inflammatory corticosteroid for ophthalmic use. Loteprednol etabonate is a white to off-white powder.Loteprednol etabonate is represented by the following struct... |
| Active Ingredient | Loteprednol etabonate |
| Dosage Form | Ointment; Gel; Suspension/drops |
| Route | Ophthalmic |
| Strength | 0.5% |
| Market Status | Prescription |
| Company | Bausch And Lomb |
A number of prescription loteprednol etabonate ophthalmic products are specifically indicated for the treatment of post-operative inflammation and pain following ocular surgery.
FDA Label
Loteprednol etabonate (LE) belongs to a unique class of corticosteroids with potent anti-inflammatory effects designed to be active at the site of action. Animal studies have shown that LE has a binding affinity to steroid receptors that is 4.3 times greater than dexamethasone. This particular class of steroids consists of bioactive molecules whose in-vivo transformation to non-toxic substances can be predicted from their chemistry and knowledge of enzymatic pathways in the body. Cortienic acid is an inactive metabolite of hydrocortisone and analogs of cortienic acid are also devoid of corticosteroid activity. Specifically, LE is an ester derivative of one of these analogs, cortienic acid etabonate. In particular, LE possesses a metabolically labile 17 beta-chloromethyl ester function which was designed in order to be hydrolyzed to an inactive carboxylic acid moiety. This inactive metabolite is more hydrophilic and is thus readily eliminated from the body. LE also exhibits good ocular permeation properties and good skin permeation properties.
Anti-Allergic Agents
Agents that are used to treat allergic reactions. Most of these drugs act by preventing the release of inflammatory mediators or inhibiting the actions of released mediators on their target cells. (From AMA Drug Evaluations Annual, 1994, p475) (See all compounds classified as Anti-Allergic Agents.)
Absorption
Loteprednol etabonate (LE) demonstrates good ocular permeation properties as it is lipid soluble, allowing the agent to penetrate into cells with relative ease. Results from the ocular administration of loteprednol in normal, healthy volunteers have shown that there are low or undetectable concentrations of either unchanged material or its metabolite. Following twice-daily unilateral topical ocular dosing of LE for 14 days in healthy subjects, the plasma concentrations of loteprednol etabonate were below the limit of quantitation (1 ng/mL) at all time points. These finds suggest that limited, if any, systemic absorption of LE occurs.
Route of Elimination
Following systemic administration to rats, loteprednol etabonate is eliminated primarily via the biliary/faecal route, with most of the dose eliminated in the form of the metabolite, PJ-90.
Volume of Distribution
The only data available regarding the volume of distribution of loteprednol etabonate (LE) is the volume of distribution the agent demonstrated when administered to dogs - a value of 3.7 L/kg. It has been shown, however, that the topical ocular administration of LE distributes preferentially into the cellular components of blood.
Clearance
Loteprednol etabonate was slowly hydrolyzed in liver at clearance rates of 0.21 +/- 0.04 and 2.41 +/- 0.13 ml/h/kg in the liver and plasma, respectively.
Loteprednol etabonate (LE) is readily and extensively metabolized to two inactive metabolites, PJ-90 (1-cortienic acid) and PJ-91 (1-cortienic acid etabonate). Metabolism occurs locally in ocular tissues, and to the extent that loteprednol etabonate reaches the systemic circulation, likely the liver and other tissues into which it distributes. In particular, studies have demonstrated that LE (chloromethyl 17alpha-ethoxycarbonyloxy-11beta-hydroxy-3-oxoandrosta-1,4-diene) is rapidly hydrolyzed at the location of its 17beta-chloromethyl ester function by paraoxonase 1 in human plasma at the site of administration at the level of the affected eye tissue to the 17beta-carboxylate PJ-91 metabolite and PJ-90 metabolite. Both metabolites are considered inactive.
The terminal half-life of loteprednol etabonate as determined when administered intravenously at a dose of 5 mg/kg in the dog animal model is 2.8 hours.
Corticosteroids like loteprednol etabonate inhibit the inflammatory response to a variety of inciting agents and likely delay or slow healing. They inhibit the edema, fibrin deposition, capillary dilation, leukocyte migration, capillary proliferation, fibroblast proliferation, deposition of collagen, and scar formation that are commonly associated with inflammation. While glucocorticoids are known to bind to and activate the glucocorticoid receptor, the molecular mechanisms involved in glucocorticoid/glucocorticoid receptor-dependent modulation of inflammation are not clearly established. Moreover, corticosteroids are thought to inhibit prostaglandin production through several independent mechanisms. In particular, corticosteroids are thought to act by the induction of phospholipase A2 inhibitory proteins, collectively called lipocortins. It is postulated that these proteins control the biosynthesis of potent mediators of inflammation such as prostaglandins and leukotrienes by inhibiting the release of their common precursor arachidonic acid. Arachidonic acid is released from membrane phospholipids by phospholipase A2. The use of LE subsequently treats post-operative inflammation and pain following ocular surgery by managing the prostaglandin release, recruitment and travel of neutrophils and macrophages, and production of other inflammatory mediators that are intrinsically associated with the physical trauma of surgery.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
89
PharmaCompass offers a list of Loteprednol Etabonate API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Loteprednol Etabonate manufacturer or Loteprednol Etabonate supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Loteprednol Etabonate manufacturer or Loteprednol Etabonate supplier.
PharmaCompass also assists you with knowing the Loteprednol Etabonate API Price utilized in the formulation of products. Loteprednol Etabonate API Price is not always fixed or binding as the Loteprednol Etabonate Price is obtained through a variety of data sources. The Loteprednol Etabonate Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Loteprednol Etabonate manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Loteprednol Etabonate, including repackagers and relabelers. The FDA regulates Loteprednol Etabonate manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Loteprednol Etabonate API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Loteprednol Etabonate manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Loteprednol Etabonate supplier is an individual or a company that provides Loteprednol Etabonate active pharmaceutical ingredient (API) or Loteprednol Etabonate finished formulations upon request. The Loteprednol Etabonate suppliers may include Loteprednol Etabonate API manufacturers, exporters, distributors and traders.
click here to find a list of Loteprednol Etabonate suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Loteprednol Etabonate DMF (Drug Master File) is a document detailing the whole manufacturing process of Loteprednol Etabonate active pharmaceutical ingredient (API) in detail. Different forms of Loteprednol Etabonate DMFs exist exist since differing nations have different regulations, such as Loteprednol Etabonate USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Loteprednol Etabonate DMF submitted to regulatory agencies in the US is known as a USDMF. Loteprednol Etabonate USDMF includes data on Loteprednol Etabonate's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Loteprednol Etabonate USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Loteprednol Etabonate suppliers with USDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Loteprednol Etabonate Drug Master File in Korea (Loteprednol Etabonate KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Loteprednol Etabonate. The MFDS reviews the Loteprednol Etabonate KDMF as part of the drug registration process and uses the information provided in the Loteprednol Etabonate KDMF to evaluate the safety and efficacy of the drug.
After submitting a Loteprednol Etabonate KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Loteprednol Etabonate API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Loteprednol Etabonate suppliers with KDMF on PharmaCompass.
A Loteprednol Etabonate written confirmation (Loteprednol Etabonate WC) is an official document issued by a regulatory agency to a Loteprednol Etabonate manufacturer, verifying that the manufacturing facility of a Loteprednol Etabonate active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Loteprednol Etabonate APIs or Loteprednol Etabonate finished pharmaceutical products to another nation, regulatory agencies frequently require a Loteprednol Etabonate WC (written confirmation) as part of the regulatory process.
click here to find a list of Loteprednol Etabonate suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Loteprednol Etabonate as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Loteprednol Etabonate API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Loteprednol Etabonate as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Loteprednol Etabonate and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Loteprednol Etabonate NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Loteprednol Etabonate suppliers with NDC on PharmaCompass.
Loteprednol Etabonate Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Loteprednol Etabonate GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Loteprednol Etabonate GMP manufacturer or Loteprednol Etabonate GMP API supplier for your needs.
A Loteprednol Etabonate CoA (Certificate of Analysis) is a formal document that attests to Loteprednol Etabonate's compliance with Loteprednol Etabonate specifications and serves as a tool for batch-level quality control.
Loteprednol Etabonate CoA mostly includes findings from lab analyses of a specific batch. For each Loteprednol Etabonate CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Loteprednol Etabonate may be tested according to a variety of international standards, such as European Pharmacopoeia (Loteprednol Etabonate EP), Loteprednol Etabonate JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Loteprednol Etabonate USP).

