
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
FDF Dossiers
0
FDA Orange Book
0
Europe
0
Canada
0
Australia
0
South Africa
0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
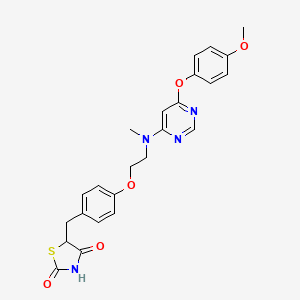

1. Ckd 501
2. Ckd-501
3. Ckd501
1. 607723-33-1
2. Lobeglitazone [inn]
3. My89f08k5d
4. 5-(4-(2-((6-(4-methoxyphenoxy)pyrimidin-4-yl)(methyl)amino)ethoxy)benzyl)thiazolidine-2,4-dione
5. Unii-my89f08k5d
6. Ckd501
7. Lobeglitazone [who-dd]
8. Schembl2742697
9. Chembl3585580
10. Chebi:136052
11. Db09198
12. Sb16869
13. 5-[[4-[2-[[6-(4-methoxyphenoxy)pyrimidin-4-yl]-methylamino]ethoxy]phenyl]methyl]-1,3-thiazolidine-2,4-dione
14. Db-090849
15. Q18350076
16. (5rs)-5((4-(2-((6-(4-methoxyphenoxy)pyrimidin-4-yl)methylamino)ethoxy)phenyl)methyl)-1,3-thiazolidine-2,4-dione
17. 2,4-thiazolidinedione, 5-((4-(2-((6-(4-methoxyphenoxy)-4-pyrimidinyl)methylamino)ethoxy)phenyl)methyl)-
| Molecular Weight | 480.5 g/mol |
|---|---|
| Molecular Formula | C24H24N4O5S |
| XLogP3 | 4.3 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 10 |
| Exact Mass | 480.14674105 g/mol |
| Monoisotopic Mass | 480.14674105 g/mol |
| Topological Polar Surface Area | 128 Ų |
| Heavy Atom Count | 34 |
| Formal Charge | 0 |
| Complexity | 670 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Lobeglitazone was approved by the Ministry of Food and Drug Safety (South Korea) in 2013, and is being monitored by postmarketing surveillance until 2019. Lobeglitazone is not approved for use by either the Food and Drug Administration (USA), Health Canada, or by the European Medicines Agency for use in the management of diabetes.
A - Alimentary tract and metabolism
A10 - Drugs used in diabetes
A10B - Blood glucose lowering drugs, excl. insulins
A10BG - Thiazolidinediones
A10BG04 - Lobeglitazone
Absorption
In rat studies, the AUC for the doses 0.5, 1, and 2 mg/kg, AUC values were determined to be 459, 514, and 481 ug min/mL respectively. Absoprtion occurs rapidly after administration, with Tmax of 67.5 and 48.8 min and a Cmax of 0.962 and 0.4.94 ug/mL following doses of 0.5 and 2 mg/kg, respectively. Absolute bioavailability after oral administration was nearly complete and apparently not affected by the dosage; 92.1% following a 0.5 mg/kg dose and 99.0% following a 2 mg/kg dose. Furthermore, the extent of LB remaining in the GI tract at 24 h was found to be negligible, with values less than 0.2% of the oral dose, suggesting that the intestinal absorption is complete in rats at the dose range studied.
Route of Elimination
It has been reported that the combined extent of the excretion of lobeglitazone to the bile, urine and intestine is low (less than 10% of total dose), suggesting that the major route of elimination for the drug involves its metabolism.
Volume of Distribution
The steady state volume of distribution (Vss) of lobeglitazone was found to be 189276 mL/kg. Vss was not found to vary statistically with the dose, suggesting that lobeglitazone follows linear kinetics.
Clearance
In rat studies, systemic clearance was found to be between 1.95 and 2.19 mL/min/kg regardless of dosage.
Rat studies with lobeglitazone have suggested that it is primarily metabolized by cytochrome P450 (CYP) isozymes, however the exact enzymes involved in its metabolism have yet to be elucidated. The structure of Lobeglitazone's five major metabolites have been characterized along with their pharmacokinetic parameters, and can be seen in the metabolism section below. In rat studies, demethylation and hydroxylation appear to be the primary metabolic pathways. The most abundant metabolite found in these studies was confirmed in vivo as M1, a demethylated derivative of lobeglitazone; its rate of formation was found to be approximately 0.216 0.252 mL/min/kg, representing approximately 9.76% of the total lobeglitazone elimination in vivo in rats.
Following an intravenous dosage of 1 mg/kg, the half life was found to be 110 min.
Lobeglitazone acts as an insulin sensitizer by binding and activating Peroxisome Proliferator-Activated Receptors (PPAR) gamma within fat cells. By promoting the binding of insulin at fat cells, lobeglitazone has been shown to reduce blood sugar levels, lower hemoglobain A1C (HbA1C) levels, and improve lipid and liver profiles. Unlike [DB01132], which is a dual PPAR agonist at PPAR-alpha and PPAR-gamma, Lobeglitazone is a pure PPAR-alpha agonist.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Market Place

ABOUT THIS PAGE
61
PharmaCompass offers a list of Lobeglitazone API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Lobeglitazone manufacturer or Lobeglitazone supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Lobeglitazone manufacturer or Lobeglitazone supplier.
PharmaCompass also assists you with knowing the Lobeglitazone API Price utilized in the formulation of products. Lobeglitazone API Price is not always fixed or binding as the Lobeglitazone Price is obtained through a variety of data sources. The Lobeglitazone Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Lobeglitazone manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Lobeglitazone, including repackagers and relabelers. The FDA regulates Lobeglitazone manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Lobeglitazone API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Lobeglitazone manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Lobeglitazone supplier is an individual or a company that provides Lobeglitazone active pharmaceutical ingredient (API) or Lobeglitazone finished formulations upon request. The Lobeglitazone suppliers may include Lobeglitazone API manufacturers, exporters, distributors and traders.
click here to find a list of Lobeglitazone suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
Lobeglitazone Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Lobeglitazone GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Lobeglitazone GMP manufacturer or Lobeglitazone GMP API supplier for your needs.
A Lobeglitazone CoA (Certificate of Analysis) is a formal document that attests to Lobeglitazone's compliance with Lobeglitazone specifications and serves as a tool for batch-level quality control.
Lobeglitazone CoA mostly includes findings from lab analyses of a specific batch. For each Lobeglitazone CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Lobeglitazone may be tested according to a variety of international standards, such as European Pharmacopoeia (Lobeglitazone EP), Lobeglitazone JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Lobeglitazone USP).

