



API Suppliers

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
0
Listed Suppliers
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
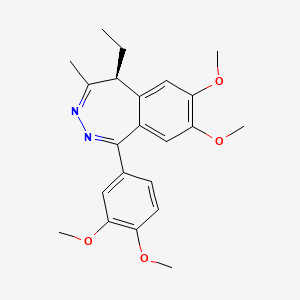

1. 1-(3,4-dimethoxyphenyl)-5-ethyl-7,8-dimethoxy-4-methyl-5h-2,3-benzodiazepine
2. Dextofisopam
3. Egyt-341
4. Grandaxin
5. Tofisopam
6. Tofizopam
1. (s)-tofisopam
2. 11zyl7qk34
3. 82059-51-6
4. (-)-(5s)-1-(3,4-dimethoxyphenyl)-5-ethyl-7,8-dimethoxy-4-methyl-5h-2,3-benzodiazepine
5. Levotofisopam [usan]
6. Levotofisopam [usan:inn]
7. Unii-11zyl7qk34
8. Levotofisopam [inn]
9. Levotofisopam (usan/inn)
10. Schembl5431173
11. Chembl2107351
12. Zinc3831552
13. D04721
14. Q27251353
15. (5s)-1-(3,4-dimethoxyphenyl)-4-methyl-5-ethyl-7,8-dimethoxy-5h-2,3-benzodiazepine
16. 5h-2,3-benzodiazepine, 1-(3,4-dimethoxyphenyl)-5-ethyl-7,8-dimethoxy-4-methyl-, (5s)-
17. 5h-2,3-benzodiazepine, 1-(3,4-dimethoxyphenyl)-5-ethyl-7,8-dimethoxy-4-methyl, (5s)-
| Molecular Weight | 382.5 g/mol |
|---|---|
| Molecular Formula | C22H26N2O4 |
| XLogP3 | 3.2 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 6 |
| Exact Mass | 382.18925731 g/mol |
| Monoisotopic Mass | 382.18925731 g/mol |
| Topological Polar Surface Area | 61.6 Ų |
| Heavy Atom Count | 28 |
| Formal Charge | 0 |
| Complexity | 579 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Antidepressive Agents
Mood-stimulating drugs used primarily in the treatment of affective disorders and related conditions. Several MONOAMINE OXIDASE INHIBITORS are useful as antidepressants apparently as a long-term consequence of their modulation of catecholamine levels. The tricyclic compounds useful as antidepressive agents (ANTIDEPRESSIVE AGENTS, TRICYCLIC) also appear to act through brain catecholamine systems. A third group (ANTIDEPRESSIVE AGENTS, SECOND-GENERATION) is a diverse group of drugs including some that act specifically on serotonergic systems. (See all compounds classified as Antidepressive Agents.)


