
Synopsis
Synopsis
0
JDMF
0
VMF
0
US Patents
0
US Exclusivities
0
Health Canada Patents
Annual Reports
NA
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Arava
2. Hwa 486
3. Hwa-486
4. Hwa486
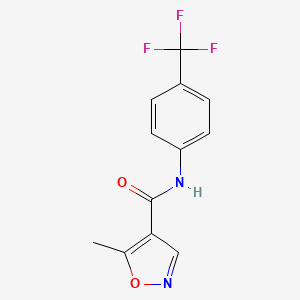
5. N-(4-trifluoromethyphenyl)-5-methylisoxazole-4-carboxamide
6. Su101
1. 75706-12-6
2. Arava
3. Lefunamide
4. Leflunomida
5. Leflunomidum
6. 5-methyl-n-[4-(trifluoromethyl)phenyl]-1,2-oxazole-4-carboxamide
7. Hwa 486
8. Hwa-486
9. Repso
10. Leflunomidum [inn-latin]
11. Leflunomide Medac
12. Su101
13. 5-methyl-n-(4-(trifluoromethyl)phenyl)isoxazole-4-carboxamide
14. Arava (tn)
15. Su-101
16. 5-methylisoxazole-4-carboxylic Acid (4-trifluoromethyl)anilide
17. Leflunomide Teva
18. 5-methyl-n-(4-(trifluoromethyl)phenyl)-4-isoxazolecarboxamide
19. 4-isoxazolecarboxamide, 5-methyl-n-[4-(trifluoromethyl)phenyl]-
20. Alpha,alpha,alpha-trifluoro-5-methyl-4-isoxazolecarboxy-p-toluidide
21. Leflunomide Winthrop
22. Sulol
23. Hwa486
24. 5-methyl-n-[4-(trifluoromethyl)phenyl]isoxazole-4-carboxamide
25. Leflunomide Ratiopharm
26. Rs-34821
27. L04aa13
28. Chembl960
29. Nsc-677411
30. Nsc-759864
31. 5-methylisoxazole-4-(4-trifluoromethylcarboxanilide)
32. Mls000069648
33. Chebi:6402
34. G162gk9u4w
35. 4-isoxazolecarboxamide, 5-methyl-n-(4-(trifluoromethyl)phenyl)-
36. Ncgc00015610-02
37. Smr000058209
38. 5-methyl-n-[4-(trifluoromethyl)phenyl]-4-isoxazolecarboxamide
39. Leflunomide 100 Microg/ml In Acetonitrile
40. Cas-75706-12-6
41. Dsstox_cid_3201
42. Dsstox_rid_76923
43. Dsstox_gsid_23201
44. Leflunomida [inn-spanish]
45. Su 101 (pharmaceutical)
46. Lefunomide [inn-spanish]
47. Leflunomide [inn]
48. Hsdb 7289
49. Sr-01000000191
50. Unii-g162gk9u4w
51. Arabloc
52. N-(4'-trifluoromethylphenyl)-5-methylisoxazole-4-carboxamide
53. Prestwick_87
54. Leflunomide [usan:usp:inn:ban]
55. Mfcd00867593
56. Su 101
57. Spectrum_000322
58. Leflunomide [mi]
59. Opera_id_1709
60. Prestwick0_000772
61. Prestwick1_000772
62. Prestwick2_000772
63. Prestwick3_000772
64. Spectrum5_000850
65. Leflunomide [jan]
66. Lopac-l-5025
67. Leflunomide [hsdb]
68. Leflunomide [usan]
69. L 5025
70. Leflunomide [vandf]
71. Schembl5057
72. Leflunomide [mart.]
73. Bidd:pxr0189
74. Lopac0_000649
75. Bspbio_000844
76. Kbioss_000802
77. Leflunomide [usp-rs]
78. Leflunomide [who-dd]
79. Leflunomide, Immunosuppressant
80. Mls001076267
81. Divk1c_000916
82. Leflunomide (jan/usp/inn)
83. Spectrum1503927
84. Spbio_002783
85. Leflunomide [ema Epar]
86. Bpbio1_000930
87. Gtpl6825
88. Zinc4840
89. Dtxsid9023201
90. Hms502n18
91. Kbio1_000916
92. Kbio2_000802
93. Kbio2_003370
94. Kbio2_005938
95. Leflunomide [ep Impurity]
96. Leflunomide [orange Book]
97. Ninds_000916
98. 4-isoxazolecarboxamide, 5-methyl-n-(4-(trifluoromethyl)phenyl
99. Hms1570k06
100. Hms1922m06
101. Hms2090o12
102. Hms2097k06
103. Hms2235c07
104. Hms3262a19
105. Hms3268d12
106. Hms3371f21
107. Hms3414p03
108. Hms3654f07
109. Hms3673m17
110. Hms3678n21
111. Hms3714k06
112. Hms3865i13
113. Leflunomide [ep Monograph]
114. Leflunomide For Peak Identification
115. Pharmakon1600-01503927
116. Leflunomide [usp Monograph]
117. Albb-019233
118. Bcp22241
119. Hy-b0083
120. Tox21_110182
121. Tox21_301873
122. Tox21_500649
123. Bdbm50054601
124. Dl-433
125. Nsc677411
126. Nsc759864
127. S1247
128. Stl426823
129. Akos000265193
130. Tox21_110182_1
131. Ac-6796
132. Bcp9000846
133. Ccg-204736
134. Cs-1781
135. Db01097
136. Ks-1076
137. Lp00649
138. Nsc 677411
139. Nsc 759864
140. Sb17287
141. Sdccgsbi-0050629.p003
142. Idi1_000916
143. Ncgc00015610-01
144. Ncgc00015610-03
145. Ncgc00015610-04
146. Ncgc00015610-05
147. Ncgc00015610-06
148. Ncgc00015610-07
149. Ncgc00015610-08
150. Ncgc00015610-09
151. Ncgc00015610-10
152. Ncgc00015610-11
153. Ncgc00015610-12
154. Ncgc00015610-13
155. Ncgc00015610-14
156. Ncgc00015610-17
157. Ncgc00015610-18
158. Ncgc00015610-30
159. Ncgc00022625-03
160. Ncgc00022625-04
161. Ncgc00022625-05
162. Ncgc00022625-06
163. Ncgc00022625-07
164. Ncgc00022625-08
165. Ncgc00255370-01
166. Ncgc00261334-01
167. Bm164612
168. A9622
169. Ab00052389
170. Eu-0100649
171. Ft-0621959
172. L0250
173. Sw196399-3
174. C07905
175. D00749
176. Mls-0003109.0001
177. Ab00052389-17
178. Ab00052389-18
179. Ab00052389_19
180. Ab00052389_21
181. 706l126
182. Q248550
183. Q-201289
184. Sr-01000000191-2
185. Sr-01000000191-4
186. Sr-01000000191-7
187. Brd-k78692225-001-03-9
188. Brd-k78692225-001-11-2
189. 5-methyl-4-(4-trifluoromethyl-phenyl)aminocarbonylisoxazole
190. 5-methyl-4-(4-trifluoromethylphenyl)aminocarbonylisoxazole
191. Leflunomide, European Pharmacopoeia (ep) Reference Standard
192. N-(4-trifluoromethylphenyl)-5-methylisoxazole-4-carboxamide
193. 5-methyl-n-[4-(trifluoromethyl)-phenyl]isoxazole-4-carboxamide
194. 5-methylisoxazole-4-carboxylic Acid (4-trifluoromethyl)-anilide
195. N-(4-trifluoromethylphenyl)-5-methylisoxa-zole-4-carboxamide
196. Isoxazole-4-carboxamide, 5-methyl-n-[4-(trifluoromethyl)phenyl]-
197. Leflunomide, United States Pharmacopeia (usp) Reference Standard
198. 5-methyl-n-(4-(trifluoromethyl)phenyl)isoxazole-4-carboxamide;leflunomide
199. Hwa486; Rs-34821; Su101;hwa 486; Rs 34821; Su 101
200. Leflunomide, Pharmaceutical Secondary Standard; Certified Reference Material
201. N-(4-(trifluoromethyl)phenyl) 5 Methylisoxazole-4-carboxamide
202. Leflunomide For Peak Identification, European Pharmacopoeia (ep) Reference Standard
| Molecular Weight | 270.21 g/mol |
|---|---|
| Molecular Formula | C12H9F3N2O2 |
| XLogP3 | 2.5 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 2 |
| Exact Mass | 270.06161202 g/mol |
| Monoisotopic Mass | 270.06161202 g/mol |
| Topological Polar Surface Area | 55.1 Ų |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 327 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Arava |
| PubMed Health | Leflunomide (By mouth) |
| Drug Classes | Immune Modulator, Immune Suppressant |
| Drug Label | ARAVA (leflunomide) is a pyrimidine synthesis inhibitor. The chemical name for leflunomide is N-(4'-trifluoromethylphenyl)-5-methylisoxazole-4-carboxamide. It has an empirical formula C12H9F3N2O2, a molecular weight of 270.2 and the following struc.. |
| Active Ingredient | Leflunomide |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 100mg; 10mg; 20mg |
| Market Status | Prescription |
| Company | Sanofi Aventis Us |
| 2 of 4 | |
|---|---|
| Drug Name | Leflunomide |
| PubMed Health | Leflunomide (By mouth) |
| Drug Classes | Immune Modulator, Immune Suppressant |
| Drug Label | Leflunomide is a pyrimidine synthesis inhibitor. The chemical name for leflunomide is N-(4-trifluoromethylphenyl)-5-methylisoxazole-4-carboxamide. It has an empirical formula C12H9F3N2O2, a molecular weight of 270.2 and the following structural fo... |
| Active Ingredient | Leflunomide |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 10mg; 20mg |
| Market Status | Prescription |
| Company | Apotex; Alembic Pharms; Sandoz; Teva Pharms; Heritage Pharms; Barr |
| 3 of 4 | |
|---|---|
| Drug Name | Arava |
| PubMed Health | Leflunomide (By mouth) |
| Drug Classes | Immune Modulator, Immune Suppressant |
| Drug Label | ARAVA (leflunomide) is a pyrimidine synthesis inhibitor. The chemical name for leflunomide is N-(4'-trifluoromethylphenyl)-5-methylisoxazole-4-carboxamide. It has an empirical formula C12H9F3N2O2, a molecular weight of 270.2 and the following struc.. |
| Active Ingredient | Leflunomide |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 100mg; 10mg; 20mg |
| Market Status | Prescription |
| Company | Sanofi Aventis Us |
| 4 of 4 | |
|---|---|
| Drug Name | Leflunomide |
| PubMed Health | Leflunomide (By mouth) |
| Drug Classes | Immune Modulator, Immune Suppressant |
| Drug Label | Leflunomide is a pyrimidine synthesis inhibitor. The chemical name for leflunomide is N-(4-trifluoromethylphenyl)-5-methylisoxazole-4-carboxamide. It has an empirical formula C12H9F3N2O2, a molecular weight of 270.2 and the following structural fo... |
| Active Ingredient | Leflunomide |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 10mg; 20mg |
| Market Status | Prescription |
| Company | Apotex; Alembic Pharms; Sandoz; Teva Pharms; Heritage Pharms; Barr |
Antirheumatic
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001., p. 971
Leflunomide is indicated to alleviate the signs and symptoms of rheumatoid arthritis and to slow joint impairment. /Included in US product labeling/
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 1758
Leflunomide, a new oral immunomodulatory agent, is effective for the treatment of rheumatoid arthritis. Its mechanism of action in suppressing inflammation is based in its inhibition of dihydroorotate dehydrogenase, an enzyme responsible for de novo synthesis of pyrimidine containing ribonucleotides. It is the first disease-modifying antirheumatic drug approved for treatment of rheumatoid arthritis with an indication for retardation of joint damage by radiography. Side effects are generally mild and include diarrhea, rashes, reversible alopecia, and elevation of hepatic transaminases. Despite the concern about hepatotoxicity, combination use with methotrexate in treating patients with rheumatoid arthritis has been shown to be safe. Other autoimmune diseases in which leflunomide has been used successfully include Felty syndrome, vasculitis, Sjogren syndrome, Wegener granulomatosis, and bullous pemphigoid.
PMID:12003373 Sanders S, Harisdangkul V; Am J Med Sci 323 (4): 190-3 (2002)
Leflunomide has excellent antiviral activity against cytomegalovirus (CMV) in animal models and is considerably less expensive than intravenous ganciclovir. We used leflunomide in four consenting renal allograft recipients with symptomatic CMV disease, who were unable to afford ganciclovir and would otherwise remain untreated. This is the first report of efficacy of leflunomide in humans with CMV disease. They received loading dose of 100 mg of leflunomide once daily on days 1-3 and then 20 mg once daily for 3 months. All four patients were followed up three times weekly with physical examination, total leukocyte counts, blood urea and serum creatinine for a minimum period of 6 weeks. None of the patients showed drug related adverse events, alteration in cyclosporine levels, or decreased graft function, except one who developed leucopenia. Preliminary data presented suggests that leflunomide therapy for CMV disease is effective and could be used with careful monitoring in allograft recipients who cannot afford intravenous ganciclovir therapy. The duration of treatment and the role of leflunomide in secondary prophylaxis and in situations of ganciclovir resistance need to be studied further.
PMID:15167608 John GT et al; Transplantation 77 (9): 1460-1 (2004)
FDA Pregnancy Risk Category: X /CONTRAINDICATED IN PREGNANCY. Studies in animals or humans, or investigational or post-marketing reports, have demonstrated positive evidence of fetal abnormalities or risk which clearly outweights any possible benefit to the patient./
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 1758
Because it can take up to 2 years for plasma concentrations of the active metabolite of leflunomide (A77 1726) to decrease to undetectable concentrations (less than 0.02 ug/mL) following discontinuance of leflunomide, the possibility that adverse effects or drug interactions associated with the drug could continue to occur even thought the patient is no longer receiving leflunomide should be considered.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2004. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2004 (Plus Supplements)., p. 3609
Opportunistic infections and serious infection, including sepsis and death, have been reported rarely in patients receiving leflunomide. Most serious infections reported in patients receiving leflunomide occurred in those receiving concomitant therapy with immunosuppressive agent and/or those with comorbid illness that, in addition to rheumatoid arthritis, could have predisposed them to infections.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2004. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2004 (Plus Supplements)., p. 3609
Leflunomide, a new immunomodulatory agent, was prescribed to a 67-year-old female patient with rheumatoid arthritis. Fifteen days later she developed diarrhea and elevated liver enzymes. A liver biopsy showed a pattern of acute hepatitis. The patient was homozygous for the rare CYP2C9*3 allele, which determines the slowest metabolic rate for CYP2C9 enzymatic activity, that is probably involved in the metabolism of leflunomide. Liver damage subsided in few weeks. This case illustrates the risk of hepatotoxicity by leflunomide and suggests that it is possibly related to CYP2C9 polymorphism.
PMID:14971821 Sevilla-Mantilla C et al; Dig Liver Dis 36 (1): 82-4 (2004)
For more Drug Warnings (Complete) data for LEFLUNOMIDE (20 total), please visit the HSDB record page.
For the management of the signs and symptoms of active rheumatoid arthritis (RA) to improve physical function and to slow the progression of structural damage associated with the disease. Has also been used for the prevention of acute and chronic rejection in recipients of solid organ trasnplants and is designated by the FDA as an orphan drug for this use.
FDA Label
Leflunomide is indicated for the treatment of adult patients with:
- active rheumatoid arthritis as a 'disease-modifying antirheumatic drug' (DMARD);
- active psoriatic arthritis .
Recent or concurrent treatment with hepatotoxic or haematotoxic DMARDs (e. g. methotrexate) may result in an increased risk of serious adverse reactions; therefore, the initiation of leflunomide treatment has to be carefully considered regarding these benefit / risk aspects.
Moreover, switching from leflunomide to another DMARD without following the washout procedure may also increase the risk of serious adverse reactions even for a long time after the switching.
Leflunomide is indicated for the treatment of adult patients with:
- active rheumatoid arthritis as a 'disease-modifying antirheumatic drug' (DMARD);
- active psoriatic arthritis .
Recent or concurrent treatment with hepatotoxic or haematotoxic DMARDs (e. g. methotrexate) may result in an increased risk of serious adverse reactions; therefore, the initiation of leflunomide treatment has to be carefully considered regarding these benefit / risk aspects.
Moreover, switching from leflunomide to another DMARD without following the washout procedure may also increase the risk of serious adverse reactions even for a long time after the switching.
Leflunomide is indicated for the treatment of adult patients with:
- active rheumatoid arthritis as a 'disease-modifying antirheumatic drug' (DMARD);
- active psoriatic arthritis .
Recent or concurrent treatment with hepatotoxic or haematotoxic DMARDs (e. g. methotrexate) may result in an increased risk of serious adverse reactions; therefore, the initiation of leflunomide treatment has to be carefully considered regarding these benefit / risk aspects.
Moreover, switching from leflunomide to another DMARD without following the washout procedure may also increase the risk of serious adverse reactions even for a long time after the switching.
Leflunomide is indicated for the treatment of adult patients with:
- active rheumatoid arthritis as a 'disease-modifying antirheumatic drug' (DMARD).
Recent or concurrent treatment with hepatotoxic or haematotoxic DMARDs (e. g. methotrexate) may result in an increased risk of serious adverse reactions, therefore, the initiation of leflunomide treatment has to be carefully considered regarding these benefit / risk aspects.
Moreover, switching from leflunomide to another DMARD without following the washout procedure may also increase the risk of serious adverse reactions even for a long time after the switching.
Leflunomide is indicated for the treatment of adult patients with:
- active rheumatoid arthritis as a disease-modifying antirheumatic drug (DMARD);
- active psoriatic arthritis .
Recent or concurrent treatment with hepatotoxic or haematotoxic DMARDs (e. g. methotrexate) may result in an increased risk of serious adverse reactions; therefore, the initiation of leflunomide treatment has to be carefully considered regarding these benefit / risk aspects.
Moreover, switching from leflunomide to another DMARD without following the washout procedure may also increase the risk of serious adverse reactions even for a long time after the switching.
Leflunomide is indicated for the treatment of adult patients with active rheumatoid arthritis as a 'disease-modifying antirheumatic drug' (DMARD).
Recent or concurrent treatment with hepatotoxic or haematotoxic DMARDs (e. g. methotrexate) may result in an increased risk of serious adverse reactions; therefore, the initiation of leflunomide treatment has to be carefully considered regarding these benefit / risk aspects.
Moreover, switching from leflunomide to another DMARD without following the washout procedure may also increase the risk of serious adverse reactions even for a long time after the switching.
Leflunomide is a pyrimidine synthesis inhibitor indicated in adults for the treatment of active rheumatoid arthritis (RA). RA is an auto-immune disease characterized by high T-cell activity. T cells have two pathways to synthesize pyrimidines: the salvage pathways and the de novo synthesis. At rest, T lymphocytes meet their metabolic requirements by the salvage pathway. Activated lymphocytes need to expand their pyrimidine pool 7- to 8-fold, while the purine pool is expanded only 2- to 3-fold. To meet the need for more pyrimidines, activated T cells use the de novo pathway for pyrimidine synthesis. Therefore, activated T cells, which are dependent on de novo pyrimidine synthesis, will be more affected by leflunomide's inhibition of dihydroorotate dehydrogenase than other cell types that use the salvage pathway of pyrimidine synthesis.
Immunosuppressive Agents
Agents that suppress immune function by one of several mechanisms of action. Classical cytotoxic immunosuppressants act by inhibiting DNA synthesis. Others may act through activation of T-CELLS or by inhibiting the activation of HELPER CELLS. While immunosuppression has been brought about in the past primarily to prevent rejection of transplanted organs, new applications involving mediation of the effects of INTERLEUKINS and other CYTOKINES are emerging. (See all compounds classified as Immunosuppressive Agents.)
Enzyme Inhibitors
Compounds or agents that combine with an enzyme in such a manner as to prevent the normal substrate-enzyme combination and the catalytic reaction. (See all compounds classified as Enzyme Inhibitors.)
L04AA13
L04AA13
L04AA13
L04AA13
L04AA13
L04AA13
L04AA13
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
L - Antineoplastic and immunomodulating agents
L04 - Immunosuppressants
L04A - Immunosuppressants
L04AA - Selective immunosuppressants
L04AA13 - Leflunomide
Absorption
Well absorbed, peak plasma concentrations appear 6-12 hours after dosing
Route of Elimination
The active metabolite is eliminated by further metabolism and subsequent renal excretion as well as by direct biliary excretion. In a 28 day study of drug elimination (n=3) using a single dose of radiolabeled compound, approximately 43% of the total radioactivity was eliminated in the urine and 48% was eliminated in the feces. It is not known whether leflunomide is excreted in human milk. Many drugs are excreted in human milk, and there is a potential for serious adverse reactions in nursing infants from leflunomide.
Volume of Distribution
0.13 L/kg
Following oral administration of leflunomide, the drug is rapidly converted to A77 1726 in the GI mucosa and liver.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2004. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2004 (Plus Supplements)., p. 3612
Time to peak concentration: Approximately 6 to 12 hours. /M1 metabolite/
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 1758
M1 metabolite is 80% bioavailable. Administration of leflunomide with a high-fat meal has no effect on the plasma concentration of M1. /M1 metabolite/
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 1758
M1 has a low volume of distribution (Vss = 0.13 L/kg) and is extensively bound (>99.3%) to albumin in healthy subjects. Protein binding has been shown to be linear at therapeutic concentrations. The free fraction of M1 is slightly higher in patients with rheumatoid arthritis and approximately doubled in patients with chronic renal failure; the mechanism and significance of these increases are unknown.
Physicians Desk Reference. 58th ed. Thomson PDR. Montvale, NJ 2004., p. 726
For more Absorption, Distribution and Excretion (Complete) data for LEFLUNOMIDE (8 total), please visit the HSDB record page.
Primarily hepatic. Leflunomide is converted to its active form following oral intake.
Leflunomide is metabolized to M1 and other minor active metabolites. An active metabolite, 4-trifluoromethylaniline, is present in plasma at low concentrations. Although the specific site of leflunomide metabolism is unknown, it has been suggested that the gastrointestinal wall and liver play a role in the metabolism.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 1758
The 3-unsubstituted isoxazole ring in the anti-inflammatory drug leflunomide undergoes a unique N-O bond cleavage to the active alpha-cyanoenol metabolite A771726, which resides in the same oxidation state as the parent. In vitro studies were conducted to characterize drug-metabolizing enzyme(s) responsible for ring opening and to gain insight into the mechanism of ring opening. ... Although A771726 formation in human liver microsomes or recombinant p4501A2 required NADPH, its formation was greatly reduced by oxygen or carbon monoxide, suggesting that the isoxazole ring opening was catalyzed by the p450Fe(II) form of the enzyme. A mechanism for the p450-mediated ring scission is proposed in which the isoxazole ring nitrogen or oxygen coordinates to the reduced form of the heme followed by charge transfer from p450Fe(II) to the C=N bond or deprotonation of the C3-H, which results in a cleavage of the N-O bond.
PMID:12975333 Kalgutkar AS et al; Drug Metab Dispos 31 (10): 1240-50 (2003)
Leflunomide has known human metabolites that include (E)-3-Hydroxy-2-methanimidoyl-N-[4-(trifluoromethyl)phenyl]but-2-enamide.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
2 weeks
2 weeks /M1 metabolite/
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 1758
Leflunomide is a prodrug that is rapidly and almost completely metabolized following oral administration to its pharmacologically active metabolite, A77 1726. This metabolite is responsible for essentially all of the drug's activity in-vivo. The mechanism of action of leflunomide has not been fully determined, but appears to primarily involve regulation of autoimmune lymphocytes. It has been suggested that leflunomide exerts its immunomodulating effects by preventing the expansion of activated autoimmune lymphocytes via interferences with cell cycle progression. In-vitro data indicates that leflunomide interferes with cell cycle progression by inhibiting dihydroorotate dehydrogenase (a mitochondrial enzyme involved in de novo pyrimidine ribonucleotide uridine monophosphate (rUMP)synthesis) and has antiproliferative activity. Human dihydroorotate dehydrogenase consists of 2 domains: an /-barrel domain containing the active site and an -helical domain that forms a tunnel leading to the active site. A77 1726 binds to the hydrophobic tunnel at a site near the flavin mononucleotide. Inhibition of dihydroorotate dehydrogenase by A77 1726 prevents production of rUMP by the de novo pathway; such inhibition leads to decreased rUMP levels, decreased DNA and RNA synthesis, inhibition of cell proliferation, and G1 cell cycle arrest. It is through this action that leflunomide inhibits autoimmune T-cell proliferation and production of autoantibodies by B cells. Since salvage pathways are expected to sustain cells arrested in the G1 phase, the activity of leflunomide is cytostatic rather than cytotoxic. Other effects that result from reduced rUMP levels include interference with adhesion of activated lymphocytes to the synovial vascular endothelial cells, and increased synthesis of immunosuppressive cytokines such as transforming growth factor- (TGF-). Leflunomide is also a tyrosine kinase inhibitor. Tyrosine kinases activate signalling pathways leading to DNA repair, apoptosis and cell proliferation. Inhibition of tyrosine kinases can help to treating cancer by preventing repair of tumor cells.
Leflunomide exhibits anit-inflammatory activity by inhibiting cyclooxygenase-2 (COX-2).
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2004. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2004 (Plus Supplements)., p. 3612
It has been suggested that leflunomide exerts its immunomodulating effects by preventing the expansion of activated autoimmune lymphocytes via interference with cell cycle progression. ... In vitro data indicate that leflunomide interferes with cell cycle progression by inhibiting the mitochondrial enzyme dihydroorotate dehydrogenase; there also is in vitro evidence that the drug inhibits protein tyrosine kinase activity in dividing cells and possesses other effect that may contribute to its immunomodulating activity.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2004. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2004 (Plus Supplements)., p. 3612
... In this study, we examined the effect of A771726 /active metabolite of leflunomide/ on osteoclast formation and bone-resorbing activity in vitro, using cultures of bone marrow-derived osteoclast progenitors and purified functionally mature osteoclasts, and then we elucidated the molecular mechanism of action of the effect of A771726 on osteoclasts. A771726 inhibited osteoclast formation from macrophage colony-stimulating factor (M-CSF)-dependent osteoclast progenitors in the presence of receptor activator of nuclear factor kappa B (NF-kappaB) ligand (RANKL), without any other types of cells present, in a dose-related manner, similar to the inhibition in cultures of unfractionated bone marrow cells. In addition, A771726 suppressed bone resorption by isolated mature osteoclasts. These results indicate that A771726 directly and intrinsically inhibited the differentiation and function of osteoclast lineage cells without any mediation by other cells. The inhibition by A771726 was not restored by the simultaneous addition of uridine, and may be independent of the blockade of NF-kappaB activation and the tyrosine phosphorylation of proteins. Thus, leflunomide, through its active metabolite, has the potential to prevent bone loss by directly inhibiting osteoclastogenesis and osteoclast function. This inhibition suggests a novel mechanism for leflunomide in the retardation of the joint destruction observed in rheumatoid arthritis patients.
PMID:15221489 Kobayashi Y et al; J Bone Miner Metab 22 (4): 318-28 (2004)

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
55
PharmaCompass offers a list of Leflunomide API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Leflunomide manufacturer or Leflunomide supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Leflunomide manufacturer or Leflunomide supplier.
PharmaCompass also assists you with knowing the Leflunomide API Price utilized in the formulation of products. Leflunomide API Price is not always fixed or binding as the Leflunomide Price is obtained through a variety of data sources. The Leflunomide Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Leflunomide manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Leflunomide, including repackagers and relabelers. The FDA regulates Leflunomide manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Leflunomide API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Leflunomide manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Leflunomide supplier is an individual or a company that provides Leflunomide active pharmaceutical ingredient (API) or Leflunomide finished formulations upon request. The Leflunomide suppliers may include Leflunomide API manufacturers, exporters, distributors and traders.
click here to find a list of Leflunomide suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Leflunomide DMF (Drug Master File) is a document detailing the whole manufacturing process of Leflunomide active pharmaceutical ingredient (API) in detail. Different forms of Leflunomide DMFs exist exist since differing nations have different regulations, such as Leflunomide USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Leflunomide DMF submitted to regulatory agencies in the US is known as a USDMF. Leflunomide USDMF includes data on Leflunomide's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Leflunomide USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Leflunomide suppliers with USDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Leflunomide Drug Master File in Korea (Leflunomide KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Leflunomide. The MFDS reviews the Leflunomide KDMF as part of the drug registration process and uses the information provided in the Leflunomide KDMF to evaluate the safety and efficacy of the drug.
After submitting a Leflunomide KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Leflunomide API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Leflunomide suppliers with KDMF on PharmaCompass.
A Leflunomide CEP of the European Pharmacopoeia monograph is often referred to as a Leflunomide Certificate of Suitability (COS). The purpose of a Leflunomide CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Leflunomide EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Leflunomide to their clients by showing that a Leflunomide CEP has been issued for it. The manufacturer submits a Leflunomide CEP (COS) as part of the market authorization procedure, and it takes on the role of a Leflunomide CEP holder for the record. Additionally, the data presented in the Leflunomide CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Leflunomide DMF.
A Leflunomide CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Leflunomide CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Leflunomide suppliers with CEP (COS) on PharmaCompass.
A Leflunomide written confirmation (Leflunomide WC) is an official document issued by a regulatory agency to a Leflunomide manufacturer, verifying that the manufacturing facility of a Leflunomide active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Leflunomide APIs or Leflunomide finished pharmaceutical products to another nation, regulatory agencies frequently require a Leflunomide WC (written confirmation) as part of the regulatory process.
click here to find a list of Leflunomide suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Leflunomide as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Leflunomide API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Leflunomide as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Leflunomide and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Leflunomide NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Leflunomide suppliers with NDC on PharmaCompass.
Leflunomide Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Leflunomide GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Leflunomide GMP manufacturer or Leflunomide GMP API supplier for your needs.
A Leflunomide CoA (Certificate of Analysis) is a formal document that attests to Leflunomide's compliance with Leflunomide specifications and serves as a tool for batch-level quality control.
Leflunomide CoA mostly includes findings from lab analyses of a specific batch. For each Leflunomide CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Leflunomide may be tested according to a variety of international standards, such as European Pharmacopoeia (Leflunomide EP), Leflunomide JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Leflunomide USP).

