
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
VMF
DRUG PRODUCT COMPOSITIONS
0
FDF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
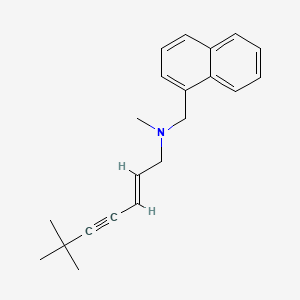

1. (e)-n-(6,6-dimethyl-2-heptenynyl)-n-methyl-1-naphthalenementhamin Hydrochloride
2. Da 5505
3. Lamisil
4. Sf 86 327
5. Sf 86-327
6. Sf 86327
7. Sf-86-327
8. Sf86327
9. Tdt 067
10. Tdt-067
11. Tdt067
12. Terbinafine Hydrochloride
13. Terbinafine, (z)-
14. Terbinafine, (z)-isomer
1. 91161-71-6
2. Lamisil
3. Lamasil
4. (e)-n,6,6-trimethyl-n-(naphthalen-1-ylmethyl)hept-2-en-4-yn-1-amine
5. Sf-86-327
6. Lamisil At
7. Sf 86-327
8. Terbinafin
9. Terbinex
10. (e)-n-(6,6-dimethyl-2-hepten-4-ynyl)-n-methyl-1-naphthalenemethylamine
11. Terbinafina
12. (e)-n-(6,6-dimethyl-2-hepten-4-ynyl)-n-methyl-1-naphthalene Methanamine
13. Terbinafine Free Base
14. Tdt-067
15. G7riw8s0xp
16. Chembl822
17. Chebi:9448
18. Lamisil Tablet
19. 91161-71-6 (free Base)
20. Terbinafinum
21. Zabel
22. 1-naphthalenemethanamine, N-(6,6-dimethyl-2-hepten-4-ynyl)-n-methyl-, (e)-
23. Ncgc00159346-02
24. (2e)-n,6,6-trimethyl-n-(1-naphthylmethyl)hept-2-en-4-yn-1-amine
25. (2e)-n,6,6-trimethyl-n-(naphthalen-1-ylmethyl)hept-2-en-4-yn-1-amine
26. Dsstox_cid_3640
27. N-[(2e)-6,6-dimethyl-2-hepten-4-yn-1-yl]-n-methyl-1-naphthalenemethanamine
28. Dsstox_rid_77123
29. Dsstox_gsid_23640
30. Lamasil (tn)
31. Cas-91161-71-6
32. Unii-g7riw8s0xp
33. Terbinafine (usan/inn)
34. Brn 4256376
35. Corbinal
36. Terbinafine [usan:inn:ban]
37. N,6,6-trimethyl-n-(1-naphthylmethyl)hept-2-en-4-yn-1-amine Hydrochloride
38. Erbinafine Hydrochloride
39. Terbinafine [mi]
40. Terbinafine, Sf-86-327, Lamisil, Tbnf
41. Terbinafine [inn]
42. Terbinafine [usan]
43. Ec 618-706-8
44. Terbinafine [vandf]
45. Terbinafine [mart.]
46. Schembl36794
47. Schembl37843
48. Terbinafine [who-dd]
49. Mls006011885
50. Bidd:gt0825
51. Dtxsid2023640
52. Terbinafine (lamisil, Terbinex)
53. Terbinafine [green Book]
54. Chebi:94705
55. Terbinafine [orange Book]
56. Hms2089b20
57. Hms3715l08
58. Albb-027269
59. Bcp22896
60. Osurnia Component Terbinafine
61. Zinc1530981
62. Tox21_111591
63. (e)-n,6,6-trimethyl-n-(1-naphthylmethyl)hept-2-en-4-yn-1-amine
64. Bbl010959
65. Bdbm50018518
66. Hy-17395a
67. Mfcd00242672
68. S1725
69. Stk802069
70. Akos001451917
71. Tox21_111591_1
72. Ac-8561
73. Ccg-221253
74. Cs-1944
75. Db00857
76. Gs-3099
77. Terbinafine Component Of Osurnia
78. (e)-n,6,6-trimethyl-n-(naphthalen-1
79. Ncgc00159346-03
80. Ncgc00159346-04
81. Ncgc00188975-01
82. Terbinafine [ema Epar Veterinary]
83. Sf86-327
84. Smr004703509
85. Sbi-0206829.p001
86. Sw197656-3
87. T3677
88. C08079
89. D02375
90. Ab00698510-07
91. Ab00698510-09
92. Ab00698510-10
93. Ab00698510-11
94. Ab00698510_12
95. Ab00698510_13
96. Ab00698510_14
97. 161t716
98. A843743
99. Q415259
100. Brd-k68132782-003-05-4
101. (6,6-dimethyl-hept-2-en-4-ynyl)-methyl-naphthalen-1-ylmethyl-amine
102. N,6,6-trimethyl-n-(1-naphthylmethyl)-2-heptene-4-yne-1-amine
103. Sf 86-327; Sf-86-327; Sf86-327; Lamasil; Lamisil At
104. ((e)-6,6-dimethyl-hept-2-en-4-ynyl)-methyl-naphthalen-1-ylmethyl-amine
105. 1n,6,6-trimethyl-1n-(1-naphthylmethyl)-(e)-2-hepten-4-yn-1-amine
106. (6,6-dimethyl-hept-2-en-4-ynyl)-methyl-naphthalen-1-ylmethyl-amine(terbinafine)
107. (6,6-dimethylhept-2-en-4-yn-1-yl)methyl(1-naphthylmethyl)amine Hydrochloride
108. [(2e)-6,6-dimethylhept-2-en-4-yn-1-yl](methyl)[(naphthalen-1-yl)methyl]amine
109. [(e)-6,6-dimethyl-hept-2-en-4-ynyl]-methyl-(naphthalen-1-yl-methyl)-amine
110. 1-naphthalenemethanamine, N-[(2e)-6,6-dimethyl-2-hepten-4-yn-1-yl]-n-methyl-
| Molecular Weight | 291.4 g/mol |
|---|---|
| Molecular Formula | C21H25N |
| XLogP3 | 5.6 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 5 |
| Exact Mass | 291.198699802 g/mol |
| Monoisotopic Mass | 291.198699802 g/mol |
| Topological Polar Surface Area | 3.2 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 428 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Lamisil |
| PubMed Health | Terbinafine |
| Drug Classes | Antifungal |
| Drug Label | Lamisil Tablets contain the synthetic allylamine antifungal compound terbinafine hydrochloride.Chemically, terbinafine hydrochloride is (E)-N-(6,6-dimethyl-2-hepten-4-ynyl)-N-methyl-1-naphthalenemethanamine hydrochloride. The empirical formula C21H26... |
| Active Ingredient | Terbinafine hydrochloride |
| Dosage Form | Tablet; Cream; Granule |
| Route | Oral; Topical |
| Strength | eq 187.5mg base/packet; 1%; eq 250mg base; eq 125mg base/packet |
| Market Status | Over the Counter; Prescription |
| Company | Novartis |
| 2 of 4 | |
|---|---|
| Drug Name | Lamisil at |
| Active Ingredient | Terbinafine hydrochloride; Terbinafine |
| Dosage Form | Spray; Gel; Solution |
| Route | Topical |
| Strength | 1% |
| Market Status | Over the Counter |
| Company | Novartis |
| 3 of 4 | |
|---|---|
| Drug Name | Lamisil |
| PubMed Health | Terbinafine |
| Drug Classes | Antifungal |
| Drug Label | Lamisil Tablets contain the synthetic allylamine antifungal compound terbinafine hydrochloride.Chemically, terbinafine hydrochloride is (E)-N-(6,6-dimethyl-2-hepten-4-ynyl)-N-methyl-1-naphthalenemethanamine hydrochloride. The empirical formula C21H26... |
| Active Ingredient | Terbinafine hydrochloride |
| Dosage Form | Tablet; Cream; Granule |
| Route | Oral; Topical |
| Strength | eq 187.5mg base/packet; 1%; eq 250mg base; eq 125mg base/packet |
| Market Status | Over the Counter; Prescription |
| Company | Novartis |
| 4 of 4 | |
|---|---|
| Drug Name | Lamisil at |
| Active Ingredient | Terbinafine hydrochloride; Terbinafine |
| Dosage Form | Spray; Gel; Solution |
| Route | Topical |
| Strength | 1% |
| Market Status | Over the Counter |
| Company | Novartis |
Terbinafine hydrochloride is indicated to treat fungal skin and nail infections caused by _Trichophyton_ species, _Microsporum canis_, _Epidermophyton floccosum_, and _Tinea_ species. Terbinafine hydrochloride also treats yeast infections of the skin caused by _Candida_ species and _Malassezia furfur_.
FDA Label
Terbinafine is an allylamine antifungal that inhibits squalene epoxidase (also known as squalene monooxygenase) to prevent the formation of ergosterol and cause an accumulation of squalene, weakening the cell wall of fungal cells. Terbinafine distributes into tissues and has a long terminal elimination half life, so the duration of action is long. Overdose with terbinafine is rare, even above the therapeutic dose, so the therapeutic index is wide. Patients taking oral terbinafine should have liver function tests performed prior to treatment to reduce the risk of liver injury.
Antifungal Agents
Substances that destroy fungi by suppressing their ability to grow or reproduce. They differ from FUNGICIDES, INDUSTRIAL because they defend against fungi present in human or animal tissues. (See all compounds classified as Antifungal Agents.)
Enzyme Inhibitors
Compounds or agents that combine with an enzyme in such a manner as to prevent the normal substrate-enzyme combination and the catalytic reaction. (See all compounds classified as Enzyme Inhibitors.)
D - Dermatologicals
D01 - Antifungals for dermatological use
D01A - Antifungals for topical use
D01AE - Other antifungals for topical use
D01AE15 - Terbinafine
D - Dermatologicals
D01 - Antifungals for dermatological use
D01B - Antifungals for systemic use
D01BA - Antifungals for systemic use
D01BA02 - Terbinafine
Absorption
Oral terbinafine is >70% absorbed but only 40% bioavailable after first pass metabolism, reaching a Cmax of 1g/mL with a Tmax of 2 hours an an AUC of 4.56g\*h/mL. Over the course of a week, 1% topical terbinafine's Cmax increases from 949-1049ng/cm2 and the AUC increases from 9694-13,492ng/cm2/h.
Route of Elimination
Terbinafine is approximately 80% eliminated in urine, while the remainder is eliminated in feces. The unmetabolized parent drug is not present in urine.
Volume of Distribution
A single 250mg oral dose of terbinafine has a volume of distribution at steady state of 947.5L or 16.6L/kg.
Clearance
A single 250mg oral dose of terbinafine has a clearance of 76L/h or 1.11L/h/kg.
Terbinafine can be deaminated to 1-naphthaldehyde by CYP2C9, 2B6, 2C8, 1A2, 3A4, and 2C19. 1-naphthaldehyde is then oxidized to 1-naphthoic acid or reduced to 1-naphthalenemethanol. Terbinafine can also be hydroxylated by CYP1A2, 2C9, 2C8, 2B6, and 2C19 to hydroxyterbinafine. Hydroxyterbinafine is then oxidized to carboxyterbinafine or N-demethylated by CYP3A4, 2B6, 1A2, 2C9, 2C8, and 2C19 to desmethylhydroxyterbinafine. Terbinafine can be N-demethylated to desmethylterbinafine. Desmethylterbinafine is then dihydroxylated to a desmethyldihydrodiol or hydroxylated to desmethylhydroxyterbinafine. Finally, terbinafine can be dihydroxylated to a dihydrodiol which is then N-demethylated to a desmethyldihydrodiol.
Terbinafine has known human metabolites that include 1-Naphtaldehyde, Hydroxyterbinafine, and N-Desmethylterbinafine.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
Oral terbinafine has an effective half life of approximately 36 hours. However, the terminal half life ranges from 200-400 hours as it distributes into skin and adipose tissue. 1% topical terbinafine's half life increases over the first seven days from approximately 10-40 hours.
Terbinafine inhibits the enzyme squalene monooxygenase (also called squalene epoxidase), preventing the conversion of squalene to 2,3-oxydosqualene, a step in the synthesis of ergosterol. This inhibition leads to decreased ergosterol, which would normally be incorporated into the cell wall, and accumulation of squalene. Generation of a large number of squalene containing vesicles in the cytoplasm may leach other lipids away from, and further weaken, the cell wall.
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 20266
Submission : 2007-01-29
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 27059
Submission : 2013-06-07
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 33058
Submission : 2019-06-18
Status : Active
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]About the Company : DKSH, founded with the goal of improving people's lives, assists businesses with market expansion and business growth in both existing and emerging markets. It has been fostering g...
About the Company : APIPL is a fully-integrated Indian pharmaceutical company manufacturing APIs for various segments. We are one of the quality manufacturers and suppliers of over a dozen APIs. These...

About the Company : HELM Portugal is HELM’s Competence Centre for the marketing of Active Pharmaceutical Ingredients (API). The leading APIs distributed by Helm include Ascorbic Acid pharma Grade (C...

About the Company : Hoventa Pharma is in the business of manufacturing APIs for over three decades.We offer various kinds of services in addition to supplies of APIs, we undertake long-term contracts ...

About the Company : We produce Active Pharmaceutical Ingredients (APIs) providing integrated full-service capabilities. Olon’s ability to develop and manufacture Active Pharmaceutical Ingredients (...

About the Company : Refarmed Chemicals is a fully integrated Swiss-based marketing company with extensive experience in the generics industry. Our global market activity and strategic offices located ...

About the Company : Shamrock Medicaments Ltd, formerly known as Doctor Lifeline Remedies India Ltd, has an intriguing evolution. Established within the robust Shamrock Group in 2001 with a hefty $35 m...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Terclara (terbinafine) is a topical formulation of terbinafine which is approved for the treatment of mild to moderate fungal infections in nail and nail bed (Onychomycosis).
Lead Product(s): Terbinafine,Inapplicable
Therapeutic Area: Infections and Infectious Diseases Brand Name: Terclara
Study Phase: Approved FDFProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable February 03, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Terbinafine,Inapplicable
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Terclara (MOB-015) is Now Being Launched in Norway
Details : Terclara (terbinafine) is a topical formulation of terbinafine which is approved for the treatment of mild to moderate fungal infections in nail and nail bed (Onychomycosis).
Product Name : Terclara
Product Type : Miscellaneous
Upfront Cash : Inapplicable
February 03, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Terclara (terbinafine) is a topical squalene monooxygenase inhibitor antifungal drug candidate, which is launched in Sweden for the treatment of onychomycosis.
Lead Product(s): Terbinafine,Inapplicable
Therapeutic Area: Infections and Infectious Diseases Brand Name: Terclara
Study Phase: Approved FDFProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable September 13, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Terbinafine,Inapplicable
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Moberg Pharma Lowers Expectations for Primary Endpoint in Phase 3 Trial
Details : Terclara (terbinafine) is a topical squalene monooxygenase inhibitor antifungal drug candidate, which is launched in Sweden for the treatment of onychomycosis.
Product Name : Terclara
Product Type : Miscellaneous
Upfront Cash : Inapplicable
September 13, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
MOB-015 is a topical formulation of terbinafine which is approved for the treatment of mild to moderate fungal infections in nail and nail bed(Onychomycosis).
Lead Product(s): Terbinafine,Inapplicable
Therapeutic Area: Infections and Infectious Diseases Brand Name: Terclara
Study Phase: Approved FDFProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable May 07, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Terbinafine,Inapplicable
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
National Approval for MOB-015 in All 13 Countries
Details : MOB-015 is a topical formulation of terbinafine which is approved for the treatment of mild to moderate fungal infections in nail and nail bed(Onychomycosis).
Product Name : Terclara
Product Type : Miscellaneous
Upfront Cash : Inapplicable
May 07, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Terclara (terbinafine) is a topical squalene monooxygenase inhibitor antifungal drug candidate, which is launched in Sweden for the treatment of onychomycosis.
Lead Product(s): Terbinafine,Inapplicable
Therapeutic Area: Infections and Infectious Diseases Brand Name: Terclara
Study Phase: Approved FDFProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable February 07, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Terbinafine,Inapplicable
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
MOB-015 Launched in Sweden as Terclara; Pharmacies Report Major Demand for New Drug
Details : Terclara (terbinafine) is a topical squalene monooxygenase inhibitor antifungal drug candidate, which is launched in Sweden for the treatment of onychomycosis.
Product Name : Terclara
Product Type : Miscellaneous
Upfront Cash : Inapplicable
February 07, 2024

Details:
Terbinafine is a Other Small Molecule drug candidate, which is currently being evaluated in phase II clinical studies for the treatment of Onychomycosis.
Lead Product(s): Terbinafine,Inapplicable
Therapeutic Area: Infections and Infectious Diseases Brand Name: Undisclosed
Study Phase: Phase IIProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable November 26, 2021

Lead Product(s) : Terbinafine,Inapplicable
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Phase II
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
To Evaluate Hallux Terbinafine Subungual Gel (HSG) in the Treatment of Onychomycosis
Details : Terbinafine is a Other Small Molecule drug candidate, which is currently being evaluated in phase II clinical studies for the treatment of Onychomycosis.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
November 26, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
The agreement with Allderma complements the existing licensing agreement for MOB-015 in Europe. Allderma is responsible for marketing, distribution and sales in Sweden, Denmark and Norway, while Moberg Pharma is responsible for the manufacture and delivery of the product.
Lead Product(s): Terbinafine,Inapplicable
Therapeutic Area: Infections and Infectious Diseases Brand Name: Terclara
Study Phase: Phase IIIProduct Type: Miscellaneous
Sponsor: Allderma
Deal Size: Undisclosed Upfront Cash: Undisclosed
Deal Type: Collaboration November 08, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Terbinafine,Inapplicable
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Allderma
Deal Size : Undisclosed
Deal Type : Collaboration
Moberg Pharma Enters Into Collaboration with Allderma for Launch in Scandinavia
Details : The agreement with Allderma complements the existing licensing agreement for MOB-015 in Europe. Allderma is responsible for marketing, distribution and sales in Sweden, Denmark and Norway, while Moberg Pharma is responsible for the manufacture and delive...
Product Name : Terclara
Product Type : Miscellaneous
Upfront Cash : Undisclosed
November 08, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Resilia is proud to offer patients an effective and safe product like Solace Cream, previously available by prescription. Solace Cream is formulated to help control eczema flares and keep future eczema flares dormant longer by utilizing essential skin building blocks.
Lead Product(s): Terbinafine,Inapplicable
Therapeutic Area: Dermatology Brand Name: Solace
Study Phase: Approved FDFProduct Type: Miscellaneous
Sponsor: Pelle Ventures
Deal Size: Undisclosed Upfront Cash: Undisclosed
Deal Type: Acquisition October 25, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Terbinafine,Inapplicable
Therapeutic Area : Dermatology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Pelle Ventures
Deal Size : Undisclosed
Deal Type : Acquisition
Resilia Pharmaceuticals Acquires License to Commercialize Solace™ Eczema Cream in the United Sta...
Details : Resilia is proud to offer patients an effective and safe product like Solace Cream, previously available by prescription. Solace Cream is formulated to help control eczema flares and keep future eczema flares dormant longer by utilizing essential skin bu...
Product Name : Solace
Product Type : Miscellaneous
Upfront Cash : Undisclosed
October 25, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
MOB-015 is our next-generation nail fungus treatment targeting both over-the-counter (OTC) and prescription markets around the world. The company's timeline remains, with planned submission for MOB-015 in Europe this year and expected market approval in 2023.
Lead Product(s): Terbinafine,Inapplicable
Therapeutic Area: Infections and Infectious Diseases Brand Name: Terclara
Study Phase: Phase IIIProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable September 22, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Terbinafine,Inapplicable
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Moberg Pharma Receives Approval from The EMA's Paediatric Committee
Details : MOB-015 is our next-generation nail fungus treatment targeting both over-the-counter (OTC) and prescription markets around the world. The company's timeline remains, with planned submission for MOB-015 in Europe this year and expected market approval in ...
Product Name : Terclara
Product Type : Miscellaneous
Upfront Cash : Inapplicable
September 22, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
The proceeds will be used for registration activities and clinical work for MOB-015. When the Rights Issue is completed, the Company intends to terminate the current Convertible Loan Agreement.
Lead Product(s): Terbinafine,Inapplicable
Therapeutic Area: Infections and Infectious Diseases Brand Name: Terclara
Study Phase: Phase IIIProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: $17.5 million Upfront Cash: Undisclosed
Deal Type: Financing November 06, 2020

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Terbinafine,Inapplicable
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : $17.5 million
Deal Type : Financing
Moberg Pharma Decides on fully Guaranteed Rights Issue of Approximately SEK 150 Million
Details : The proceeds will be used for registration activities and clinical work for MOB-015. When the Rights Issue is completed, the Company intends to terminate the current Convertible Loan Agreement.
Product Name : Terclara
Product Type : Miscellaneous
Upfront Cash : Undisclosed
November 06, 2020

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
MOB-015's superior mycological cure (percentage of patients who were fungus free) has now been demonstrated in two pivotal studies, providing further support for the company's target to make MOB-015 the future market leader in onychomycosis.
Lead Product(s): Terbinafine,Inapplicable
Therapeutic Area: Infections and Infectious Diseases Brand Name: Terclara
Study Phase: Phase IIIProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable October 14, 2020

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Terbinafine,Inapplicable
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Moberg Pharma Intends to Submit a Registration Application in Europe in 2021
Details : MOB-015's superior mycological cure (percentage of patients who were fungus free) has now been demonstrated in two pivotal studies, providing further support for the company's target to make MOB-015 the future market leader in onychomycosis.
Product Name : Terclara
Product Type : Miscellaneous
Upfront Cash : Inapplicable
October 14, 2020

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code :
Brand Name : LAMISIL
Dosage Form : GEL;TOPICAL
Dosage Strength : 1%
Approval Date : 1998-04-29
Application Number : 20846
RX/OTC/DISCN : DISCN
RLD : No
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code :
Brand Name : LAMISIL AT
Dosage Form : GEL;TOPICAL
Dosage Strength : 1%
Approval Date : 2006-07-24
Application Number : 21958
RX/OTC/DISCN : OTC
RLD : Yes
TE Code :

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ANALYTICAL

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]ABOUT THIS PAGE
19
PharmaCompass offers a list of Terbinafine API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Terbinafine manufacturer or Terbinafine supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Terbinafine manufacturer or Terbinafine supplier.
PharmaCompass also assists you with knowing the Terbinafine API Price utilized in the formulation of products. Terbinafine API Price is not always fixed or binding as the Terbinafine Price is obtained through a variety of data sources. The Terbinafine Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Lamisil Tablet manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Lamisil Tablet, including repackagers and relabelers. The FDA regulates Lamisil Tablet manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Lamisil Tablet API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Lamisil Tablet manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Lamisil Tablet supplier is an individual or a company that provides Lamisil Tablet active pharmaceutical ingredient (API) or Lamisil Tablet finished formulations upon request. The Lamisil Tablet suppliers may include Lamisil Tablet API manufacturers, exporters, distributors and traders.
click here to find a list of Lamisil Tablet suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Lamisil Tablet DMF (Drug Master File) is a document detailing the whole manufacturing process of Lamisil Tablet active pharmaceutical ingredient (API) in detail. Different forms of Lamisil Tablet DMFs exist exist since differing nations have different regulations, such as Lamisil Tablet USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Lamisil Tablet DMF submitted to regulatory agencies in the US is known as a USDMF. Lamisil Tablet USDMF includes data on Lamisil Tablet's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Lamisil Tablet USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Lamisil Tablet suppliers with USDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Lamisil Tablet Drug Master File in Korea (Lamisil Tablet KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Lamisil Tablet. The MFDS reviews the Lamisil Tablet KDMF as part of the drug registration process and uses the information provided in the Lamisil Tablet KDMF to evaluate the safety and efficacy of the drug.
After submitting a Lamisil Tablet KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Lamisil Tablet API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Lamisil Tablet suppliers with KDMF on PharmaCompass.
A Lamisil Tablet written confirmation (Lamisil Tablet WC) is an official document issued by a regulatory agency to a Lamisil Tablet manufacturer, verifying that the manufacturing facility of a Lamisil Tablet active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Lamisil Tablet APIs or Lamisil Tablet finished pharmaceutical products to another nation, regulatory agencies frequently require a Lamisil Tablet WC (written confirmation) as part of the regulatory process.
click here to find a list of Lamisil Tablet suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Lamisil Tablet as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Lamisil Tablet API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Lamisil Tablet as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Lamisil Tablet and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Lamisil Tablet NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Lamisil Tablet suppliers with NDC on PharmaCompass.
Lamisil Tablet Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Lamisil Tablet GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Lamisil Tablet GMP manufacturer or Lamisil Tablet GMP API supplier for your needs.
A Lamisil Tablet CoA (Certificate of Analysis) is a formal document that attests to Lamisil Tablet's compliance with Lamisil Tablet specifications and serves as a tool for batch-level quality control.
Lamisil Tablet CoA mostly includes findings from lab analyses of a specific batch. For each Lamisil Tablet CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Lamisil Tablet may be tested according to a variety of international standards, such as European Pharmacopoeia (Lamisil Tablet EP), Lamisil Tablet JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Lamisil Tablet USP).
