



API Suppliers

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
0
Listed Suppliers
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
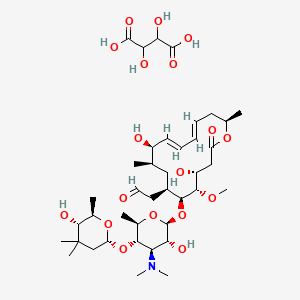

1. 2-((4r,5s,6s,7r,9r,10r,11e,13e,16r)-6-(((2s,3r,4r,5s,6r)-4-(dimethylamino)-3-hydroxy-5-(((2r,5s,6r)-5-hydroxy-4,4,6-trimethyltetrahydro-2h-pyran-2-yl)oxy)-6-methyltetrahydro-2h-pyran-2-yl)oxy)-4,10-dihydroxy-5-methoxy-9,16-dimethyl-2-oxooxacyclohexadeca-11,13-dien-7-yl)acetaldehyde 2,3-dihydroxysuccinate
2. Einecs 253-442-3
3. Unii-ipn75md9dr
4. Ipn75md9dr
5. Kitasamycin Tartrate [jan]
6. Leucomycin, (r-(r*,r*))-2,3-dihydroxybutanedioate (salt)
7. 2,3-dihydroxybutanedioic Acid;2-[(4r,5s,6s,7r,9r,10r,11e,13e,16r)-6-[(2s,3r,4r,5s,6r)-4-(dimethylamino)-3-hydroxy-5-[(2r,5s,6r)-5-hydroxy-4,4,6-trimethyloxan-2-yl]oxy-6-methyloxan-2-yl]oxy-4,10-dihydroxy-5-methoxy-9,16-dimethyl-2-oxo-1-oxacyclohexadeca-11,13-dien-7-yl]acetaldehyde
| Molecular Weight | 850.0 g/mol |
|---|---|
| Molecular Formula | C40H67NO18 |
| Hydrogen Bond Donor Count | 8 |
| Hydrogen Bond Acceptor Count | 19 |
| Rotatable Bond Count | 11 |
| Exact Mass | 849.43581429 g/mol |
| Monoisotopic Mass | 849.43581429 g/mol |
| Topological Polar Surface Area | 289 Ų |
| Heavy Atom Count | 59 |
| Formal Charge | 0 |
| Complexity | 1230 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 15 |
| Undefined Atom Stereocenter Count | 2 |
| Defined Bond Stereocenter Count | 2 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |


