
Synopsis
Synopsis
0
KDMF
0
VMF
0
Australia
0
South Africa
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
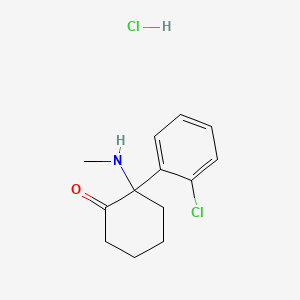

1. 2-(2-chlorophenyl)-2-(methylamino)cyclohexanone
2. Calipsol
3. Calypsol
4. Ci 581
5. Ci-581
6. Ci581
7. Kalipsol
8. Ketalar
9. Ketamine
10. Ketanest
11. Ketaset
1. 1867-66-9
2. Vetalar
3. Ketaset
4. Ketalar
5. Ketamine Hcl
6. Ketanest
7. Ketolar
8. 2-(2-chlorophenyl)-2-(methylamino)cyclohexanone Hydrochloride
9. Cl 369
10. Cn-52,372-2
11. (+-)-ketamine Hydrochloride
12. Ci-581
13. Ketamine Hydrochloride Ciii
14. Ketamine (as Hydrochloride)
15. O18yuo0i83
16. Calipsol
17. Kalipsol
18. Ketavet
19. (+-)-2-(o-chlorophenyl)-2-(methylamino)cyclohexanone Hydrochloride
20. Cl-369
21. Dea No. 7285
22. Cn-523722
23. 2-(2-chlorophenyl)-2-(methylamino)cyclohexan-1-one;hydrochloride
24. Cn-52372-2
25. Ketamine Chloride
26. Ketavet 100
27. Ketavet (veterinary)
28. Ci 581
29. Chebi:650657
30. Einecs 217-484-6
31. Unii-o18yuo0i83
32. Ketalar (tn)
33. Ketamini Hydrochloridum
34. Ketamine Hydrochloride [usan:usp:jan]
35. Cyclohexanone, 2-(2-chlorophenyl)-2-(methylamino)-, Hydrochloride
36. Cyclohexanone, 2-(2-chlorophenyl)-2-(methylamino)-, Hydrochloride, (+-)-
37. Ec 217-484-6
38. Chembl1714
39. Schembl26999
40. Dtxsid4040137
41. Ketamine Hydrochloride [mi]
42. 2-(2-chlorophenyl)-2-(methylamino)cyclohexan-1-one Hydrochloride
43. Bcp25943
44. Ketamine Hydrochloride (jp17/usp)
45. Ketamine Hydrochloride [jan]
46. (+/-)-2-(o-chlorophenyl)-2-(methylamino)cyclohexanone Hydrochloride
47. Ketamine Hydrochloride [usan]
48. Nsc116131
49. (+/-)-ketamine Hydrochloride, Solid
50. Ketamine Hydrochloride [vandf]
51. Akos005287313
52. Cyclohexanone, 2-(o-chlorophenyl)-2-(methylamino)-, Hydrochloride, (+-)-
53. Ketamine Hydrochloride [mart.]
54. Ab02117
55. Ketamine Hydrochloride [who-dd]
56. Ketamine Hydrochloride [who-ip]
57. Nsc-116131
58. Ketamine Hydrochloride [green Book]
59. Db-044614
60. Db-048467
61. Ketamine Hydrochloride [ep Impurity]
62. Ketamine Hydrochloride [orange Book]
63. Ketamine Hydrochloride [ep Monograph]
64. Ketamine Hydrochloride Ciii [usp-rs]
65. Ketamine Hydrochloride [usp Monograph]
66. Ketamini Hydrochloridum [who-ip Latin]
67. C07843
68. D00711
69. 867k669
70. A813079
71. Q-201266
72. Q27105184
73. Ketamine Hydrochloride 1.0 Mg/ml In Methanol (as Free Base)
74. 2-(2-chlorophenyl)-2-(methylamino)-1-cyclohexanone Hydrochloride
75. Ketamine Hydrochloride, European Pharmacopoeia (ep) Reference Standard
76. Cyclohexanone, 2-(2-chlorophenyl)-2-(methylamino)-, Hydrochloride (1:1)
77. Cyclohexanone, 2-(o-chlorophenyl)-2-(methylamino)-, Hydrochloride, (+/-)-
78. Ketamine (+-)-2-(o-chlorophenyl)-2-(methylamino)cyclohexanonehydrochloride
79. Ketamine Hydrochloride Solution, 1.0 Mg/ml In Methanol (as Free Base), Ampule Of 1 Ml, Certified Reference Material
| Molecular Weight | 274.18 g/mol |
|---|---|
| Molecular Formula | C13H17Cl2NO |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 2 |
| Exact Mass | 273.0687196 g/mol |
| Monoisotopic Mass | 273.0687196 g/mol |
| Topological Polar Surface Area | 29.1 Ų |
| Heavy Atom Count | 17 |
| Formal Charge | 0 |
| Complexity | 269 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 4 | |
|---|---|
| Drug Name | Ketalar |
| Drug Label | Ketalar is a nonbarbiturate anesthetic chemically designated dl 2-(0-chlorophenyl)-2-(methylamino) cyclohexanone hydrochloride. It is formulated as a slightly acid (pH 3.5-5.5) sterile solution for intravenous or intramuscular injection in concentrat... |
| Active Ingredient | Ketamine hydrochloride |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 10mg base/ml; eq 50mg base/ml; eq 100mg base/ml |
| Market Status | Prescription |
| Company | Par Sterile Products |
| 2 of 4 | |
|---|---|
| Drug Name | Ketamine hydrochloride |
| PubMed Health | Ketamine (Injection) |
| Drug Label | Ketamine hydrochloride is a nonbarbiturate anesthetic chemically designated dl 2-(0-chlorophenyl)-2-(methylamino) cyclohexanone hydrochloride. It is formulated as a slightly acid (pH 3.5-5.5) sterile solution for intravenous or intramuscular injectio... |
| Active Ingredient | Ketamine hydrochloride |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 10mg base/ml; eq 50mg base/ml; eq 100mg base/ml |
| Market Status | Prescription |
| Company | Hospira; Mylan Institutional; Eurohlth Intl |
| 3 of 4 | |
|---|---|
| Drug Name | Ketalar |
| Drug Label | Ketalar is a nonbarbiturate anesthetic chemically designated dl 2-(0-chlorophenyl)-2-(methylamino) cyclohexanone hydrochloride. It is formulated as a slightly acid (pH 3.5-5.5) sterile solution for intravenous or intramuscular injection in concentrat... |
| Active Ingredient | Ketamine hydrochloride |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 10mg base/ml; eq 50mg base/ml; eq 100mg base/ml |
| Market Status | Prescription |
| Company | Par Sterile Products |
| 4 of 4 | |
|---|---|
| Drug Name | Ketamine hydrochloride |
| PubMed Health | Ketamine (Injection) |
| Drug Label | Ketamine hydrochloride is a nonbarbiturate anesthetic chemically designated dl 2-(0-chlorophenyl)-2-(methylamino) cyclohexanone hydrochloride. It is formulated as a slightly acid (pH 3.5-5.5) sterile solution for intravenous or intramuscular injectio... |
| Active Ingredient | Ketamine hydrochloride |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 10mg base/ml; eq 50mg base/ml; eq 100mg base/ml |
| Market Status | Prescription |
| Company | Hospira; Mylan Institutional; Eurohlth Intl |
Anesthetics, Dissociative
Intravenous anesthetics that induce a state of sedation, immobility, amnesia, and marked analgesia. Subjects may experience a strong feeling of dissociation from the environment. The condition produced is similar to NEUROLEPTANALGESIA, but is brought about by the administration of a single drug. (From Gilman et al., Goodman and Gilman's The Pharmacological Basis of Therapeutics, 8th ed) (See all compounds classified as Anesthetics, Dissociative.)
Analgesics
Compounds capable of relieving pain without the loss of CONSCIOUSNESS. (See all compounds classified as Analgesics.)
Excitatory Amino Acid Antagonists
Drugs that bind to but do not activate excitatory amino acid receptors, thereby blocking the actions of agonists. (See all compounds classified as Excitatory Amino Acid Antagonists.)
 Seqens is an integrated global leader in pharmaceutical solutions & specialty ingredients, & custom-made solutions to our customers.
Seqens is an integrated global leader in pharmaceutical solutions & specialty ingredients, & custom-made solutions to our customers.
 Click Us!
Click Us! 
GDUFA
DMF Review : Reviewed
Rev. Date : 2019-11-05
Pay. Date : 2019-10-28
DMF Number : 7686
Submission : 1988-09-22
Status : Active
Type : II

Certificate Number : CEP 2005-281 - Rev 02
Issue Date : 2024-03-11
Type : Chemical
Substance Number : 1020
Status : Valid

Registration Number : 218MF10980
Registrant's Address : Raiffeisenstrasse 4, D-77933 Lahr Germany
Initial Date of Registration : 2006-12-01
Latest Date of Registration :

NDC Package Code : 49169-1041
Start Marketing Date : 2024-01-01
End Marketing Date : 2026-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

| Available Reg Filing : ASMF, CA |

 LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.

NDC Package Code : 54382-114
Start Marketing Date : 1989-02-08
End Marketing Date : 2026-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT
 Supriya Lifesciences is a cGMP-compliant API manufacturing organization with a leadership position across key & niche products.
Supriya Lifesciences is a cGMP-compliant API manufacturing organization with a leadership position across key & niche products.

GDUFA
DMF Review : Reviewed
Rev. Date : 2022-12-22
Pay. Date : 2022-12-20
DMF Number : 27921
Submission : 2014-01-15
Status : Active
Type : II

Certificate Number : CEP 2016-194 - Rev 01
Issue Date : 2024-05-07
Type : Chemical
Substance Number : 1020
Status : Valid

Date of Issue : 2025-09-02
Valid Till : 2028-07-02
Written Confirmation Number : WC-0218
Address of the Firm :

NDC Package Code : 61281-8000
Start Marketing Date : 2013-07-08
End Marketing Date : 2026-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

| Available Reg Filing : ASMF |

 Tenatra connects Indian manufacturers with global buyers through active partners in Germany, Switzerland, Belgium, Spain & Turkey.
Tenatra connects Indian manufacturers with global buyers through active partners in Germany, Switzerland, Belgium, Spain & Turkey.

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 37014
Submission : 2022-03-29
Status : Active
Type : II

Certificate Number : CEP 2022-157 - Rev 00
Issue Date : 2024-03-04
Type : Chemical
Substance Number : 1020
Status : Valid

Date of Issue : 2024-02-12
Valid Till : 2027-02-11
Written Confirmation Number : WC-0407
Address of the Firm :

NDC Package Code : 70600-035
Start Marketing Date : 2022-03-22
End Marketing Date : 2026-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

| Available Reg Filing : BR |

 Seqens is an integrated global leader in pharmaceutical solutions & specialty ingredients, & custom-made solutions to our customers.
Seqens is an integrated global leader in pharmaceutical solutions & specialty ingredients, & custom-made solutions to our customers.
 Supriya Lifesciences is a cGMP-compliant API manufacturing organization with a leadership position across key & niche products.
Supriya Lifesciences is a cGMP-compliant API manufacturing organization with a leadership position across key & niche products.
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 5462
Submission : 1984-07-17
Status : Inactive
Type : II


 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
 Seqens is an integrated global leader in pharmaceutical solutions & specialty ingredients, & custom-made solutions to our customers.
Seqens is an integrated global leader in pharmaceutical solutions & specialty ingredients, & custom-made solutions to our customers.
GDUFA
DMF Review : Complete
Rev. Date : 2019-11-05
Pay. Date : 2019-10-28
DMF Number : 7686
Submission : 1988-09-22
Status : Active
Type : II
 Supriya Lifesciences is a cGMP-compliant API manufacturing organization with a leadership position across key & niche products.
Supriya Lifesciences is a cGMP-compliant API manufacturing organization with a leadership position across key & niche products.
GDUFA
DMF Review : Complete
Rev. Date : 2022-12-22
Pay. Date : 2022-12-20
DMF Number : 27921
Submission : 2014-01-15
Status : Active
Type : II
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 37014
Submission : 2022-03-29
Status : Active
Type : II
GDUFA
DMF Review : Complete
Rev. Date : 2016-04-06
Pay. Date : 2015-04-16
DMF Number : 29094
Submission : 2015-04-14
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 19082
Submission : 2005-12-16
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 5462
Submission : 1984-07-17
Status : Inactive
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2021-06-09
Pay. Date : 2021-04-29
DMF Number : 35385
Submission : 2020-12-30
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 22256
Submission : 2008-11-28
Status : Inactive
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Seqens is an integrated global leader in pharmaceutical solutions & specialty ingredients, & custom-made solutions to our customers.
Seqens is an integrated global leader in pharmaceutical solutions & specialty ingredients, & custom-made solutions to our customers.
About the Company : Founded in 2003, Seqens has evolved into a global leader in pharmaceutical solutions and specialty ingredients. The company supports customers in the development, scale-up, and man...
 LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
About the Company : LGM Pharma is a global leader in sourcing hard-to-find APIs and intermediates for pharmaceutical and biotech industries. LGM also operates as a full-service CDMO, offering formulat...
About the Company : Virupaksha Organics, founded in 2003, is a leading manufacturer of APIs and intermediates. Its FDA-audited, ISO-certified facilities in Kazipally and Pashamylaram produce high-qual...
 Supriya Lifesciences is a cGMP-compliant API manufacturing organization with a leadership position across key & niche products.
Supriya Lifesciences is a cGMP-compliant API manufacturing organization with a leadership position across key & niche products.
About the Company : Supriya Lifescience Limited, established in 1987 and headquartered in Mumbai, is a globally recognized, technology-driven manufacturer of APIs, CDMO, and formulations. Its faciliti...
 Tenatra connects Indian manufacturers with global buyers through active partners in Germany, Switzerland, Belgium, Spain & Turkey.
Tenatra connects Indian manufacturers with global buyers through active partners in Germany, Switzerland, Belgium, Spain & Turkey.
About the Company : Tenatra International was established as a proprietorship firm in 1999. It got off to a very good start, supporting clients in the United States, Mexico and Europe. As business opp...
About the Company : Maithri Drugs Pvt. Ltd. is a global supplier of Active Pharmaceutical Ingredients (APIs), serving pharmaceutical companies in 60+ countries. Its API portfolio spans antivirals, ant...
About the Company : Arevipharma GmbH is a modern manufacturer of active pharmaceutical ingredients and intermediates with more than 140 years of expertise. Our customers are generic and research-based...

About the Company : Arevipharma GmbH is a modern manufacturer of active pharmaceutical ingredients and intermediates with more than 140 years of expertise. Our customers are generic and research-based...

About the Company : BIOTECHNICA DWC LLC has carved a niche for itself in providing value added compliance, regulatory qualification, project management and GDP guidance services to pharma companies al...

About the Company : SB Pharma GmbH, with its headquarters in Cologne, is a leading company that has set itself the goal of offering pharmaceuticals, medical products and medical devices as well as a b...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Marketed
Registration Country : Norway
Brand Name : Ketalar
Dosage Form : Solution For Injection
Dosage Strength : 10mg/ml
Packaging :
Approval Date :
Application Number :
Regulatory Info : Marketed
Registration Country : Norway

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Deregistered
Registration Country : Sweden
Brand Name : Ketanest-S
Dosage Form : Injectable Solution
Dosage Strength : 5mg/ml
Packaging :
Approval Date : 19-05-2000
Application Number : 2.00E+13
Regulatory Info : Deregistered
Registration Country : Sweden

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Prescription
Registration Country : Denmark
Brand Name : Ketador Vet.
Dosage Form : Injection Solution
Dosage Strength : 100mg/ml
Packaging :
Approval Date : 02-01-2013
Application Number : 28104999411
Regulatory Info : Prescription
Registration Country : Denmark

Regulatory Info : Authorised
Registration Country : Malta
Brand Name : Ketamine Renaudin
Dosage Form : Solution For Injection
Dosage Strength : 50MG/ML
Packaging :
Approval Date : 2024-02-05
Application Number :
Regulatory Info : Authorised
Registration Country : Malta

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Regulatory Info : Marketed
Registration Country : Norway
Brand Name : Ketamin Abcur
Dosage Form : Solution For Injection
Dosage Strength : 50mg/ml
Packaging :
Approval Date :
Application Number :
Regulatory Info : Marketed
Registration Country : Norway

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Regulatory Info : Not Marketed
Registration Country : Norway
Brand Name : Ketamine Le vet
Dosage Form : Solution For Injection
Dosage Strength : 100mg/ml
Packaging :
Approval Date :
Application Number :
Regulatory Info : Not Marketed
Registration Country : Norway

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Regulatory Info : Marketed
Registration Country : Norway
Brand Name : Ketexx Vet
Dosage Form : Solution For Injection
Dosage Strength : 100mg/ml
Packaging :
Approval Date :
Application Number :
Regulatory Info : Marketed
Registration Country : Norway

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Regulatory Info : Prescription
Registration Country : Denmark
Brand Name : Aniketam Vet.
Dosage Form : Injection Solution
Dosage Strength : 100mg/ml
Packaging :
Approval Date : 24-09-2014
Application Number : 28105440113
Regulatory Info : Prescription
Registration Country : Denmark

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Regulatory Info : Prescription
Registration Country : Denmark
Brand Name : Ketamin \"Abcur\"
Dosage Form : Injection Solution
Dosage Strength : 10mg/ml
Packaging :
Approval Date : 15-02-2016
Application Number : 28105493214
Regulatory Info : Prescription
Registration Country : Denmark

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Regulatory Info : Prescription
Registration Country : Denmark
Brand Name : Ketaminol Vet.
Dosage Form : Injection Solution
Dosage Strength : 50mg/ml
Packaging :
Approval Date : 13-08-1991
Application Number : 28101364689
Regulatory Info : Prescription
Registration Country : Denmark

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Supriya Lifesciences is a cGMP-compliant API manufacturing organization with a leadership position across key & niche products.
Supriya Lifesciences is a cGMP-compliant API manufacturing organization with a leadership position across key & niche products.
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Injection
Dosage Strength : 10MG/ML
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India
 Supriya Lifesciences is a cGMP-compliant API manufacturing organization with a leadership position across key & niche products.
Supriya Lifesciences is a cGMP-compliant API manufacturing organization with a leadership position across key & niche products.
Packaging :
Regulatory Info :
Dosage : Injection
Dosage Strength : 10MG/ML
Brand Name :
Approval Date :
Application Number :
Registration Country : India
 Supriya Lifesciences is a cGMP-compliant API manufacturing organization with a leadership position across key & niche products.
Supriya Lifesciences is a cGMP-compliant API manufacturing organization with a leadership position across key & niche products.
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Injection
Dosage Strength : 50MG/ML
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India
 Supriya Lifesciences is a cGMP-compliant API manufacturing organization with a leadership position across key & niche products.
Supriya Lifesciences is a cGMP-compliant API manufacturing organization with a leadership position across key & niche products.
Packaging :
Regulatory Info :
Dosage : Injection
Dosage Strength : 50MG/ML
Brand Name :
Approval Date :
Application Number :
Registration Country : India
 Supriya Lifesciences is a cGMP-compliant API manufacturing organization with a leadership position across key & niche products.
Supriya Lifesciences is a cGMP-compliant API manufacturing organization with a leadership position across key & niche products.
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Injection
Dosage Strength : 100MG/ML
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India
 Supriya Lifesciences is a cGMP-compliant API manufacturing organization with a leadership position across key & niche products.
Supriya Lifesciences is a cGMP-compliant API manufacturing organization with a leadership position across key & niche products.
Packaging :
Regulatory Info :
Dosage : Injection
Dosage Strength : 100MG/ML
Brand Name :
Approval Date :
Application Number :
Registration Country : India
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Vial
Dosage Strength : 50MG/ML
Packaging : 1 ml, 10 ml
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging : 1 ml, 10 ml
Regulatory Info :
Dosage : Vial
Dosage Strength : 50MG/ML
Brand Name :
Approval Date :
Application Number :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Injection
Dosage Strength : 20MG/ML
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info :
Dosage : Injection
Dosage Strength : 20MG/ML
Brand Name :
Approval Date :
Application Number :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generic
Registration Country : South Korea
Brand Name : Mortorin
Dosage Form : INJECTION
Dosage Strength : 576.8MG/10ML
Packaging :
Approval Date :
Application Number :
Regulatory Info : Generic
Registration Country : South Korea

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info : Generic
Dosage : INJECTION
Dosage Strength : 576.8MG/10ML
Brand Name : Mortorin
Approval Date :
Application Number :
Registration Country : South Korea

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Liquid Injection
Dosage Strength : 50MG/ML
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info :
Dosage : Liquid Injection
Dosage Strength : 50MG/ML
Brand Name :
Approval Date :
Application Number :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Injectable
Dosage Strength : 50MG/10ML
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info :
Dosage : Injectable
Dosage Strength : 50MG/10ML
Brand Name :
Approval Date :
Application Number :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : India
Brand Name : Ketmin 2 Vial
Dosage Form : Injection
Dosage Strength : 50MG/ML
Packaging : 10x2
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging : 10x2
Regulatory Info :
Dosage : Injection
Dosage Strength : 50MG/ML
Brand Name : Ketmin 2 Vial
Approval Date :
Application Number :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generic
Registration Country : India
Brand Name :
Dosage Form : INJECTION
Dosage Strength : 10MG/ML
Packaging : 10ml ; 20ml vial
Approval Date :
Application Number : 76092
Regulatory Info : Generic
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging : 10ml ; 20ml vial
Regulatory Info : Generic
Dosage : INJECTION
Dosage Strength : 10MG/ML
Brand Name :
Approval Date :
Application Number : 76092
Registration Country : India

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Dosage Form : Injectable / Parenteral
Grade : Parenteral, Oral, Topical
Dosage Form : Emulsion
Grade : Parenteral
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Dosage Form : Cream / Lotion / Ointment
Grade : Topical and Oral
Application : Topical
Excipients Web Link
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Excipients by Applications
Dosage Form : Emulsion, Injectable / Parenteral
Grade : Parenteral
Category : Emulsifying Agents, Parenteral, Solubilizers
Application : Parenteral
Excipient Details : Used as an osmolarity agent in culture media, tonicity adjuster in ophthalmics and parenterals solutions.
Pharmacopoeia Ref : On Request
Technical Specs : Low bacteria endotoxins, low bioburden (TAMC/TYMC). Customised pa...
Ingredient(s) : Sodium Chloride Excipient
Dosage Form : Cream / Lotion / Ointment, Gel, Injectable / Parenteral, Softgels
Grade : Parenteral, Oral, Topical
Category : Film Formers & Plasticizers, Parenteral, Thickeners and Stabilizers, Topical
Dosage Form : Capsule, Cream / Lotion / Ointment
Grade : Topical and Oral
Category : Film Formers & Plasticizers, Topical
Application : Film Formers & Plasticizers, Topical
Excipients Web Link
Brand Name : Sodium Chloride USP
Application : Parenteral
Excipient Details : A & C's Sodium Chloride is an excipient meeting the USP monograph.
Excipients Web Link
Brand Name : NaCl Multi-compendial Low Endotoxin
Application : Parenteral
Excipient Details : A & C's Sodium Chloride multi-compendial low endotoxin is an excipient meeting USP-NF, EP, BP and JP monographs.
Pharmacopoeia Ref : Multi-compendial
Technical Specs : Low Endotoxin
Ingredient(s) : Sodium Chloride Excipient
Excipients Web Link
Dosage Form : Emulsion, Injectable / Parenteral
Grade : Parenteral
Category : Emulsifying Agents, Parenteral, Solubilizers
Dosage Form : Cream / Lotion / Ointment, Gel, Solution
Grade : Oral, Topical
Category : Emulsifying Agents, Solubilizers, Topical
Application : Emulsifying Agents, Solubilizers, Topical
Excipient Details : Hydrosol 50 is used as a solubilizer and emulsifying agent in oral and topical liquid and semi-solid dosage forms.
Pharmacopoeia Ref : USP/NF
Technical Specs : N/A
Ingredient(s) : Polyoxyl 40 Hydrogenated Castor Oil
Brand Name : BENZETHONIUM CHLORIDE USP
Application : Solubilizers
Excipient Details : A & C's Benzethonium Chloride USP is a hygroscopic excipient, which meets USP requirements.
Pharmacopoeia Ref : USP
Technical Specs : 17% USP NF; 50% NF; USP
Ingredient(s) : Benzethonium Chloride Exc
Excipients Web Link
Dosage Form : Capsule, Emulsion, Softgel Capsule, Solution, Tablet, Topical Film
Grade : Not Available
Category : Film Formers & Plasticizers, Solubilizers, Topical
Application : Film Formers & Plasticizers, Solubilizers, Topical
Excipient Details : Liquid plasticizer with high ADI, hydrophilic solvent & humectant in emulsions, skin penetration enhancer in topical formulaitons.
Pharmacopoeia Ref : Ph. Eur., JP, FCC, USP
Technical Specs : Not Available
Ingredient(s) : Propylene Glycol
Global Sales Information

Dosage Form : Ketamine 100Mg 2Ml 5 Units Paren...
Dosage Strength : 5 vials 2ml 50 mg/ml
Price Per Pack (Euro) : 24.58
Published in :
Country : Italy
RX/OTC/DISCN : Class H

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Market Place

Reply
13 Apr 2024
Reply
04 Jan 2024
Reply
08 Dec 2023
Reply
13 Nov 2020
Reply
21 Aug 2019
Reply
18 Jul 2019
Reply
13 Jun 2019
Reply
13 Oct 2018
Reply
09 Oct 2018
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
REF. STANDARDS & IMPURITIES

ANALYTICAL

Product Code : NR30S05-2546WT
Classification : Optical isomers
Product Characteristics : N020605F
Category :
Description :

ABOUT THIS PAGE
86
PharmaCompass offers a list of Ketamine Hydrochloride API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Ketamine Hydrochloride manufacturer or Ketamine Hydrochloride supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Ketamine Hydrochloride manufacturer or Ketamine Hydrochloride supplier.
PharmaCompass also assists you with knowing the Ketamine Hydrochloride API Price utilized in the formulation of products. Ketamine Hydrochloride API Price is not always fixed or binding as the Ketamine Hydrochloride Price is obtained through a variety of data sources. The Ketamine Hydrochloride Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Ketavet (Veterinary) manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Ketavet (Veterinary), including repackagers and relabelers. The FDA regulates Ketavet (Veterinary) manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Ketavet (Veterinary) API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Ketavet (Veterinary) manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Ketavet (Veterinary) supplier is an individual or a company that provides Ketavet (Veterinary) active pharmaceutical ingredient (API) or Ketavet (Veterinary) finished formulations upon request. The Ketavet (Veterinary) suppliers may include Ketavet (Veterinary) API manufacturers, exporters, distributors and traders.
click here to find a list of Ketavet (Veterinary) suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Ketavet (Veterinary) DMF (Drug Master File) is a document detailing the whole manufacturing process of Ketavet (Veterinary) active pharmaceutical ingredient (API) in detail. Different forms of Ketavet (Veterinary) DMFs exist exist since differing nations have different regulations, such as Ketavet (Veterinary) USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Ketavet (Veterinary) DMF submitted to regulatory agencies in the US is known as a USDMF. Ketavet (Veterinary) USDMF includes data on Ketavet (Veterinary)'s chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Ketavet (Veterinary) USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Ketavet (Veterinary) suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Ketavet (Veterinary) Drug Master File in Japan (Ketavet (Veterinary) JDMF) empowers Ketavet (Veterinary) API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Ketavet (Veterinary) JDMF during the approval evaluation for pharmaceutical products. At the time of Ketavet (Veterinary) JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Ketavet (Veterinary) suppliers with JDMF on PharmaCompass.
A Ketavet (Veterinary) CEP of the European Pharmacopoeia monograph is often referred to as a Ketavet (Veterinary) Certificate of Suitability (COS). The purpose of a Ketavet (Veterinary) CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Ketavet (Veterinary) EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Ketavet (Veterinary) to their clients by showing that a Ketavet (Veterinary) CEP has been issued for it. The manufacturer submits a Ketavet (Veterinary) CEP (COS) as part of the market authorization procedure, and it takes on the role of a Ketavet (Veterinary) CEP holder for the record. Additionally, the data presented in the Ketavet (Veterinary) CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Ketavet (Veterinary) DMF.
A Ketavet (Veterinary) CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Ketavet (Veterinary) CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Ketavet (Veterinary) suppliers with CEP (COS) on PharmaCompass.
A Ketavet (Veterinary) written confirmation (Ketavet (Veterinary) WC) is an official document issued by a regulatory agency to a Ketavet (Veterinary) manufacturer, verifying that the manufacturing facility of a Ketavet (Veterinary) active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Ketavet (Veterinary) APIs or Ketavet (Veterinary) finished pharmaceutical products to another nation, regulatory agencies frequently require a Ketavet (Veterinary) WC (written confirmation) as part of the regulatory process.
click here to find a list of Ketavet (Veterinary) suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Ketavet (Veterinary) as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Ketavet (Veterinary) API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Ketavet (Veterinary) as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Ketavet (Veterinary) and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Ketavet (Veterinary) NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Ketavet (Veterinary) suppliers with NDC on PharmaCompass.
Ketavet (Veterinary) Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Ketavet (Veterinary) GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Ketavet (Veterinary) GMP manufacturer or Ketavet (Veterinary) GMP API supplier for your needs.
A Ketavet (Veterinary) CoA (Certificate of Analysis) is a formal document that attests to Ketavet (Veterinary)'s compliance with Ketavet (Veterinary) specifications and serves as a tool for batch-level quality control.
Ketavet (Veterinary) CoA mostly includes findings from lab analyses of a specific batch. For each Ketavet (Veterinary) CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Ketavet (Veterinary) may be tested according to a variety of international standards, such as European Pharmacopoeia (Ketavet (Veterinary) EP), Ketavet (Veterinary) JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Ketavet (Veterinary) USP).

