



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
Other Suppliers

USA (Orange Book)

Europe

Canada

Australia

South Africa
0
Uploaded Dossiers
U.S. Medicaid
Annual Reports
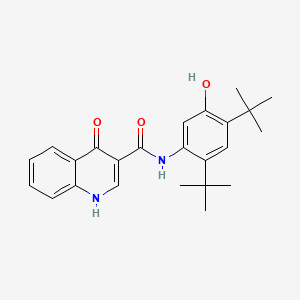

1. 3-quinolinecarboxamide, N-(2,4-bis(1,1-dimethylethyl)-5-hydroxyphenyl)-1,4-dihydro-4-oxo-
2. Kalydeco
3. N-(2,4-bis(1,1-dimethylethyl)-5-hydroxyphenyl)-4-oxo-1,4-dihydroquinoline-3-carboxamide
4. N-(2,4-di-tert-butyl-5-hydroxyphenyl)-4-oxo-1,4-dihydroquinoline-3-carboxamide
5. Vx-770
1. 873054-44-5
2. Vx-770
3. Kalydeco
4. Ivacaftor (vx-770)
5. N-(2,4-di-tert-butyl-5-hydroxyphenyl)-4-oxo-1,4-dihydroquinoline-3-carboxamide
6. Vx 770
7. N-[2,4-bis(tert-butyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxo-3-quinolinecarboxamide
8. Chebi:66901
9. N-(2,4-ditert-butyl-5-hydroxyphenyl)-4-oxo-1h-quinoline-3-carboxamide
10. 1y740ill1z
11. 1413431-05-6
12. 3-quinolinecarboxamide, N-(2,4-bis(1,1-dimethylethyl)-5-hydroxyphenyl)-1,4-dihydro-4-oxo-
13. N-(2,4-bis(1,1-dimethylethyl)-5-hydroxyphenyl)-4-oxo-1,4-dihydroquinoline-3-carboxamide
14. Vx770
15. 3-quinolinecarboxamide, N-[2,4-bis(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxo-
16. Kalydeco (tn)
17. Ivacaftor [usan]
18. Ivacaftor [usan:inn]
19. Ivacaftorum
20. Unii-1y740ill1z
21. Ivacaftor D18
22. Vx7
23. Ivacaftor [inn]
24. Ivacaftor [mi]
25. Ivacaftor (usan/inn)
26. Vx-770 - Ivacaftor
27. Ivacaftor [vandf]
28. Ivacaftor [who-dd]
29. Mls006011119
30. Schembl351373
31. Gtpl4342
32. Ivacaftor [orange Book]
33. Vx-770, Ivacaftor, Kalydeco
34. Chembl2010601
35. Dtxsid00236281
36. Ex-a441
37. Orkambi Component Ivacaftor
38. Symkevi Component Ivacaftor
39. Bcpp000199
40. Hms3654e10
41. Hms3744k05
42. Trikafta Component Ivacaftor
43. Bcp19794
44. Bdbm50032693
45. Mfcd17171361
46. S1144
47. Zinc52509463
48. Ivacaftor Component Of Orkambi
49. Akos015994762
50. Akos032950001
51. Ivacaftor Component Of Trikafta
52. Bcp9000799
53. Ccg-268562
54. Cs-0497
55. Db08820
56. Ex-7211
57. Le-0002
58. Sb16815
59. Ncgc00242480-01
60. Ncgc00242480-03
61. Ac-28324
62. Hy-13017
63. Smr004702900
64. Ft-0696681
65. Sw219620-1
66. Ec-000.2478
67. A25626
68. D09916
69. Ab01565806_02
70. Q6095693
71. Ctp-656; Ctp-656; Ctp-656; D9-ivacaftor;vx-561
72. Cystic Fibrosis Transmembrane Conductance Regulator Potentiator
73. N-(5-hydroxy-2,4-ditert-butyl-phenyl)-4-oxo-1h-quinoline-3-carboxamide
74. N-(2,4-ditert-butyl-5-hydroxyphenyl)-4-oxo-1,4-dihydroquinoline-3-carboxamide
75. N-(5-hydroxy-2,4-bis(2-methyl-2-propanyl)phenyl]-4-oxo-1,4-dihydro-3-quinolinecarboxamide
76. N-[2,4-bis(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide
77. 1134822-00-6
78. Ivacaftor;n-(2,4-di-tert-butyl-5-hydroxyphenyl)-4-oxo-1,4-dihydroquinoline-3-carboxamide;ivacaftor
| Molecular Weight | 392.5 g/mol |
|---|---|
| Molecular Formula | C24H28N2O3 |
| XLogP3 | 5.6 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 4 |
| Exact Mass | 392.20999276 g/mol |
| Monoisotopic Mass | 392.20999276 g/mol |
| Topological Polar Surface Area | 78.4 Ų |
| Heavy Atom Count | 29 |
| Formal Charge | 0 |
| Complexity | 671 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Kalydeco |
| PubMed Health | Ivacaftor (By mouth) |
| Drug Classes | Respiratory Agent |
| Drug Label | The active ingredient in KALYDECO tablets is ivacaftor, which has the following chemical name: N-(2,4-di-tert-butyl-5-hydroxyphenyl)-1,4-dihydro-4-oxoquinoline-3-carboxamide. Its molecular formula is C24H28N2O3 and its molecular weight is 392.49. Iva... |
| Active Ingredient | Ivacaftor |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 150mg |
| Market Status | Prescription |
| Company | Vertex Pharms |
| 2 of 2 | |
|---|---|
| Drug Name | Kalydeco |
| PubMed Health | Ivacaftor (By mouth) |
| Drug Classes | Respiratory Agent |
| Drug Label | The active ingredient in KALYDECO tablets is ivacaftor, which has the following chemical name: N-(2,4-di-tert-butyl-5-hydroxyphenyl)-1,4-dihydro-4-oxoquinoline-3-carboxamide. Its molecular formula is C24H28N2O3 and its molecular weight is 392.49. Iva... |
| Active Ingredient | Ivacaftor |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 150mg |
| Market Status | Prescription |
| Company | Vertex Pharms |
When used as monotherapy as the product Kalydeco, ivacaftor is indicated for the management of CF in patients age 2 years and older who have a mutation in the CFTR gene that is responsive to ivacaftor potentiation. Ivacaftor received expanded approval in May 2017 for the following 33 CFTR mutations: E56K, P67L, R74W, D110E, D110H, R117C, R117H, G178R, E193K, L206W, R347H, R352Q, A455E, S549N, S549R, G551D, G551S, D579G, S945L, S977F, F1052V, K1060T, A1067T, G1069R, R1070Q, R1070W, F1074L, D1152H, G1244E, S1251N, S1255P, D1270N, and G1349D. When used in combination with the drug [lumacaftor] as the product Orkambi, ivacaftor is indicated for the management of CF patients age 6 years and older who are shown to be homozygous for the F508del mutation in the CFTR gene. When used in combination with [tezacaftor] in the product Symdeko, it is used to manage CF in patients 12 years and older who have at least one mutation in the CFTR gene or patients aged 12 or older who are shown to be homozygous for the F508del mutation. When used in combination with tezacaftor and [elexacaftor] in the product Trikafta, it is indicated for the treatment of cystic fibrosis in patients 12 years of age and older who have at least one _F508del_ mutation in the CFTR gene.
FDA Label
Kalydeco tablets are indicated:
- As monotherapy for the treatment of adults, adolescents, and children aged 6 years and older and weighing 25 kg or more with cystic fibrosis (CF) who have an R117H CFTR mutation or one of the following gating (class III) mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene: G551D, G1244E, G1349D, G178R, G551S, S1251N, S1255P, S549N or S549R (see sections 4. 4 and 5. 1).
- In a combination regimen with tezacaftor/ivacaftor tablets for the treatment of adults, adolescents, and children aged 6 years and older with cystic fibrosis (CF) who are homozygous for the F508del mutation or who are heterozygous for the F508del mutation and have one of the following mutations in the CFTR gene: P67L, R117C, L206W, R352Q, A455E, D579G, 711+3AG, S945L, S977F, R1070W, D1152H, 2789+5GA, 3272 26AG, and 3849+10kbCT.
- In a combination regimen with ivacaftor/tezacaftor/elexacaftor tablets for the treatment of adults, adolescents, and children aged 6 years and older with cystic fibrosis (CF) who have at least one F508del mutation in the CFTR gene (see section 5. 1).
Kalydeco granules are indicated for the treatment of infants aged at least 4 months, toddlers and children weighing 5 kg to less than 25 kg with cystic fibrosis (CF) who have an R117H CFTR mutation or one of the following gating (class III) mutations in the CFTR gene: G551D, G1244E, G1349D, G178R, G551S, S1251N, S1255P, S549N or S549R (see sections 4. 4 and 5. 1).
The use of Ivacaftor has been shown to both improve CF symptoms and modulate underlying disease pathology. This is achieved by potentiating the channel opening probability (or gating) of CFTR protein in patients with impaired gating mechanisms. This is in contrast to [DB09280], another CF medication, that functions by preventing misfolding of the CFTR protein and thereby results in increased processing and trafficking of mature protein to the cell surface. Results from clinical trials indicated that treatment with ivacaftor results in improved lung function, reduced chance of experiencing a pulmonary exacerbation, reduced sweat chloride, increased weight gain, and improvements in CF symptoms and quality of life. When combined with tezacaftor, significant improvements in lung function have been observed in clinical studies. Ivacaftor was not found to increase the QTc interval in a clinically significant manner. Although ivacaftor given alone has not shown any significant improvements in patients with the delta-F508 mutation, it has shown significant improvements (>10% increase in FEV1 from baseline) in lung function for the following mutations: E56K, P67L, R74W, D110E, D110H, R117C, R117H, G178R, E193K, L206W, R347H, R352Q, A455E, S549N, S549R, G551D, G551S, D579G, S945L, S977F, F1052V, K1060T, A1067T, G1069R, R1070Q, R1070W, F1074L, D1152H, G1244E, S1251N, S1255P, D1270N, and G1349. This list was expanded by the FDA in May 2017 from 10 to 33 to accommodate more rare mutations. It is important to note that this drug may cause an increase in liver transaminases (ALT, AST). Ensure to assess liver transaminases before the initiation of treatment, every 3 months during the first year of administration, followed by every year thereafter.
Chloride Channel Agonists
A class of drugs that stimulate chloride ion influx through cell membrane channels. (See all compounds classified as Chloride Channel Agonists.)
R07AX02
R07AX30
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
R - Respiratory system
R07 - Other respiratory system products
R07A - Other respiratory system products
R07AX - Other respiratory system products
R07AX02 - Ivacaftor
Absorption
Ivacaftor is well absorbed in the gastrointestinal tract. Following administration of ivacaftor with fat-containing foods, peak plasma concentrations were reached at 4 hours (Tmax) with a maximum concentration (Cmax) of 768 ng/mL and AUC of 10600 ng * hr/mL. It is recommended that ivacaftor is taken with fat-containing foods as they increase absorption by approximately 2.5- to 4-fold.
Route of Elimination
After oral administration, ivacaftor is mainly eliminated in the feces after metabolic conversion and this elimination represents 87.8% of the dose. From the total eliminated dose, the metabolites M1 and M6 account for the majority of the eliminated dose, being 22% for M1 and 43% for M6. Ivacaftor shows negligible urinary excretion as the unchanged drug.
Volume of Distribution
After oral administration of 150 mg every 12 hours for 7 days to healthy volunteers in a fed state, the mean (SD) for apparent volume of distribution was 353 (122) L.
Clearance
The CL/F (SD) for the 150 mg dose was 17.3 (8.4) L/hr in healthy subjects.
Ivacaftor is extensively metabolized in humans. In vitro and clinical studies indicate that ivacaftor is primarily metabolized by CYP3A. From this metabolism, the major formed metabolites are M1 and M6. M1 is considered pharmacologically active even though it just presents approximately one-sixth the effect of the parent compound ivacaftor. On the other hand, M6 is not considered pharmacologically active as it represents less than one-fiftieth of the effect of the parent compound.
In a clinical study, the apparent terminal half-life was approximately 12 hours following a single dose of ivacaftor. One source mentions the half-life ranges from 12 to 14 hours.
A wide variety of CFTR mutations correlate to the Cystic Fibrosis phenotype and are associated with differing levels of disease severity. The most common mutation, affecting approximately 70% of patients with CF worldwide, is known as F508del-CFTR or delta-F508 (F508), in which a deletion in the amino acid phenylalanine at position 508 results in impaired production of the CFTR protein, thereby causing a significant reduction in the amount of ion transporter present on cell membranes. Ivacaftor as monotherapy has failed to show a benefit for patients with delta-F508 mutations, most likely due to an insufficient amount of protein available at the cell membrane for interaction and potentiation by the drug. The next most common mutation, G551D, affecting 4-5% of CF patients worldwide is characterized as a missense mutation, whereby there is sufficient amount of protein at the cell surface, but opening and closing mechanisms of the channel are altered. Ivacaftor is indicated for the management of CF in patients with this second type of mutation, as it binds to and potentiates the channel opening ability of CFTR proteins on the cell membrane. Ivacaftor exerts its effect by acting as a potentiator of the CFTR protein, an ion channel involved in the transport of chloride and sodium ions across cell membranes of the lungs, pancreas, and other organs. Alterations in the CFTR gene result in altered production, misfolding, or function of the protein and consequently abnormal fluid and ion transport across cell membranes. Ivacaftor improves CF symptoms and underlying disease pathology by potentiating the channel open probability (or gating) of CFTR protein in patients with impaired CFTR gating mechanisms. The overall level of ivacaftor-mediated CFTR chloride transport is dependent on the amount of CFTR protein at the cell surface and how responsive a particular mutant CFTR protein is to ivacaftor potentiation.






