


API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
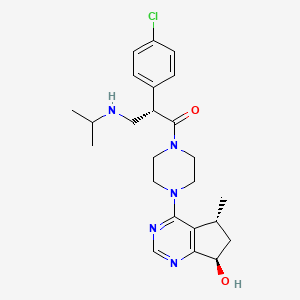

1. 2-(4-chlorophenyl)-1-(4-(7-hydroxy-5-methyl-6,7-dihydro-5h-cyclopenta(d)pyrimidin-4-yl)piperazin-1-yl)-3-(isopropylamino)propan-1-one
2. Gdc-0068
3. Gdc0068
1. Gdc-0068
2. 1001264-89-6
3. Ipatasertib (gdc-0068)
4. Rg7440
5. Rg-7440
6. Gdc0068
7. Gdc 0068
8. Gdc-0068 (rg7440)
9. 524y3ib4hq
10. (s)-2-(4-chlorophenyl)-1-(4-((5r,7r)-7-hydroxy-5-methyl-6,7-dihydro-5h-cyclopenta[d]pyrimidin-4-yl)piperazin-1-yl)-3-(isopropylamino)propan-1-one
11. Chembl2177390
12. Rg 7440
13. (2s)-2-(4-chlorophenyl)-1-(4-((5r,7r)-7-hydroxy-5-methyl-6,7-dihydro-5h-cyclopenta(d)pyrimidin-4-yl)piperazin-1-yl(-3-((propan-2-yl)amino)propan-1-one
14. (2s)-2-(4-chlorophenyl)-1-[4-[(5r,7r)-6,7-dihydro-7-hydroxy-5-methyl-5h-cyclopentapyrimidin-4-yl]-1-piperazinyl]-3-[(1-methylethyl)amino]-1-propanone
15. (2s)-2-(4-chlorophenyl)-1-[4-[(5r,7r)-7-hydroxy-5-methyl-6,7-dihydro-5h-cyclopenta[d]pyrimidin-4-yl]piperazin-1-yl]-3-(propan-2-ylamino)propan-1-one
16. 1-propanone, 2-(4-chlorophenyl)-1-(4-((5r,7r)-6,7-dihydro-7-hydroxy-5-methyl-5h-cyclopentapyrimidin-4-yl)-1-piperazinyl)-3-((1-methylethyl)amino)-, (2s)-
17. (2s)-2-(4-chlorophenyl)-1-{4-[(5r,7r)-7-hydroxy-5-methyl-6,7-dihydro-5h-cyclopenta[d]pyrimidin-4-yl]piperazin-1-yl}-3-(propan-2-ylamino)propan-1-one
18. Ipatasertib [inn]
19. Ipatasertib [usan:inn]
20. Unii-524y3ib4hq
21. Mfcd22124514
22. 1-propanone, 2-(4-chlorophenyl)-1-[4-[(5r,7r)-6,7-dihydro-7-hydroxy-5-methyl-5h-cyclopentapyrimidin-4-yl]-1-piperazinyl]-3-[(1-methylethyl)amino]-, (2s)-
23. Gdc0068 Di-hcl
24. Ipatasertib (usan/inn)
25. Ipatasertib [usan]
26. Gdc-0068 Di-hcl
27. Ipatasertib; Gdc-0068
28. Rg-7440 Di-hcl
29. Ipatasertib [who-dd]
30. Schembl191659
31. Gtpl7887
32. Chebi:95089
33. Dtxsid101025595
34. Ex-a2077
35. Bdbm50398379
36. Nsc767898
37. Nsc781451
38. Nsc800986
39. Nsc832484
40. S2808
41. Zinc68250459
42. Akos025396463
43. Bcp9000712
44. Ccg-269312
45. Cs-0975
46. Db11743
47. Nsc-767898
48. Nsc-781451
49. Nsc-800986
50. Nsc-832484
51. Ncgc00346714-01
52. 2-(4-chlorophenyl)-1-(4-(7-hydroxy-5-methyl-6,7-dihydro-5h-cyclopenta(d)pyrimidin-4-yl)piperazin-1-yl)-3-(isopropylamino)propan-1-one
53. Ac-28420
54. As-17027
55. Hy-15186
56. Bcp0726000195
57. J3.478.537f
58. D10641
59. Q27078088
60. (2s)-2-(4-chlorophenyl)-1-[4-[(5r,7r)-7-hydroxy-5-methyl-6,7-dihydro-5h-cyclopenta[e]pyrimidin-4-yl]piperazin-1-yl]-3-(propan-2-ylamino)propan-1-one
61. 0rf
| Molecular Weight | 458.0 g/mol |
|---|---|
| Molecular Formula | C24H32ClN5O2 |
| XLogP3 | 2.5 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 6 |
| Exact Mass | 457.2244530 g/mol |
| Monoisotopic Mass | 457.2244530 g/mol |
| Topological Polar Surface Area | 81.6 Ų |
| Heavy Atom Count | 32 |
| Formal Charge | 0 |
| Complexity | 622 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Treatment of breast cancer , Treatment of prostate cancer
Antineoplastic Agents
Substances that inhibit or prevent the proliferation of NEOPLASMS. (See all compounds classified as Antineoplastic Agents.)
Protein Kinase Inhibitors
Agents that inhibit PROTEIN KINASES. (See all compounds classified as Protein Kinase Inhibitors.)


