
Synopsis
Synopsis
0
KDMF
0
VMF
Annual Reports
NA
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
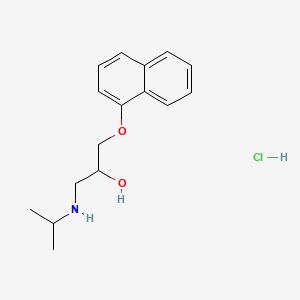

1. Anaprilin
2. Anapriline
3. Avlocardyl
4. Ay 20694
5. Ay-20694
6. Ay20694
7. Betadren
8. Dexpropranolol
9. Dociton
10. Hydrochloride, Propranolol
11. Inderal
12. Obsidan
13. Obzidan
14. Propanolol
15. Propranolol
16. Rexigen
1. 318-98-9
2. Propranolol Hcl
3. Inderal
4. Avlocardyl
5. Dociton
6. Inderalici
7. Anaprilin
8. Caridolol
9. 3506-09-0
10. Berkolol
11. Herzbase
12. Indobloc
13. Naprilin
14. Pronovan
15. Pylapron
16. Sloprolol
17. Apsolol
18. Beprane
19. Deralin
20. Ikopal
21. Obsidan
22. Tesnol
23. Kemi
24. Propranolol.hcl
25. Beta Neg
26. Innopran Xl
27. Propranolol Chloride
28. Inderex
29. Nsc-91523
30. Hemangeol
31. Innopran
32. Propranolol (hydrochloride)
33. Propanolol Hydrochloride
34. Ici 45520
35. 1-(isopropylamino)-3-(1-naphthyloxy)-2-propanol Hydrochloride
36. Ay 64043
37. 1-(isopropylamino)-3-(naphthalen-1-yloxy)propan-2-ol Hydrochloride
38. Ici-45520
39. Propranolol Hydrochloride (+/-)
40. Ay-64043
41. I 2065
42. 1-naphthalen-1-yloxy-3-(propan-2-ylamino)propan-2-ol;hydrochloride
43. (s)-propranolol (hydrochloride)
44. 1-(isopropylamino)-3-(1-naphthoxy)-propan-2-ol Hydrochloride
45. (+/-)-propranolol Hydrochloride
46. F8a3652h1v
47. Hemangiol
48. Bedranol
49. Inderol
50. Pranolol
51. Sumial
52. Indermigran
53. Proprahexal
54. Propranovitan
55. Angilol
56. Arcablock
57. Artensol
58. Biocard
59. Blocaryl
60. Cardinol
61. Corbeta
62. Detensol
63. Dibudinate
64. Dumopranol
65. Efectolol
66. Emforal
67. Farprolol
68. Frekven
69. Hemipralon
70. Kidoral
71. Nelderal
72. Noloten
73. Novopranol
74. Panolol
75. Prandol
76. Propabloc
77. Propadex
78. Propalong
79. Propayerst
80. Prophylux
81. Propral
82. Propranur
83. Rapynogen
84. Sagittol
85. Sawatol
86. Scandrug
87. Sudenol
88. Tensiflex
89. Tiperal
90. Acifol
91. Cinlol
92. Ciplar
93. Elbrol
94. Herzul
95. Oposim
96. Pranix
97. Procor
98. Prosin
99. Nedis
100. Sinal
101. Tonum
102. 1-naphthalen-1-yloxy-3-(propan-2-ylamino)propan-2-ol Hydrochloride
103. 318-98-9 (hcl)
104. Half-inderal
105. Prano-puren
106. Propra Vt Ct
107. Dsstox_cid_1198
108. Inderal Hydrochloride
109. Inderal La
110. Proberta La
111. Pur-bloka
112. Beta-neg
113. Dsstox_rid_76007
114. Dsstox_gsid_21198
115. (s)-propranolol Hydrochloride
116. 1-isopropylamino-3-(1-naphthoxy)-propan-2-ol-hydrochloride
117. Dl-propranolol Hydrochloride
118. 1-(1-naphthyloxy)-2-hydroxy-3-isopropylaminopropane Hydrochloride
119. R+c5989educor
120. (+-)-1-(isopropylamino)-3-(1-naphthyloxy)-2-propanol Hydrochloride
121. 2-propanol, 1-((1-methylethyl)amino)-3-(1-naphthalenyloxy)-, Hydrochloride
122. Propraratiopharm
123. Beta-tablinen
124. Beta-timelets
125. Dl-anapriline
126. (+/-)-1-(isopropylamino)-3-(1-naphthyloxy)-2-propanol Hydrochloride
127. [2-hydroxy-3-(naphthalen-1-yloxy)propyl](propan-2-yl)amine Hydrochloride
128. 1-[(1-methylethyl)amino]-3-(naphthalen-1-yloxy)propan-2-ol Hydrochloride
129. Chebi:8500
130. Smr000059167
131. Nsc91523
132. Ccris 1105
133. Hsdb 3176
134. Sr-01000075285
135. (r)-propranolol Hydrochloride
136. Einecs 206-268-7
137. Mfcd00012558
138. D,l-propranolol Hydrochloride
139. Servanolol
140. Efektolol
141. Migrastat
142. Unii-f8a3652h1v
143. Propranolol Hydrochloride Intensol
144. Dl-propranolol Hcl
145. Relax-b
146. Einecs 222-501-5
147. Inderal (tn)
148. Nsc 91523
149. Betacap
150. Monoprolol
151. Innopran Xl (tn)
152. (r)-(+)-propanolol Hydrochloride
153. (r)-propranolol Hcl
154. Propranolol Hydrochloride [usan:usp:jan]
155. Propranolon Hydrochloride
156. 1-(isopropylamino)-3-(1-naphthyloxy)propan-2-ol Hydrochloride
157. 1-(isopropylamino)-3-(alpha-naphthoxy)-2-propanol Hydrochloride
158. Cas-318-98-9
159. Chembl1671
160. Schembl41688
161. (y)-propranolol Hydrochloride
162. Mls000859628
163. Mls001055449
164. Mls001056753
165. Mls002222321
166. Mls002548855
167. (?)-propranolol Hydrochloride
168. Spectrum1505270
169. Propranolol Hydrochloride, 99%
170. Dtxsid3021198
171. (2-hydroxy-3-(naphthyloxy)propyl)isopropylammonium Chloride
172. Hms1570p06
173. Hms1571g12
174. Hms1571i04
175. Hms1922p19
176. Pharmakon1600-01505270
177. Act02695
178. Bcp15350
179. Bcp16675
180. Eur-1000
181. Hy-b0573
182. Kdm-1102
183. Rac-propranolol Hydrochloride
184. Tox21_201886
185. Tox21_302905
186. Tox21_500896
187. 2-propanol, 1-(isopropylamino)-3-(1-naphthyloxy)-, Hydrochloride
188. Ccg-39239
189. Ccg-39447
190. Ncs-91523
191. Nsc758950
192. S4076
193. Propranalol Hydrochloride, Dl-
194. Propranolol Hydrochloride [mi]
195. 1-(naphthalen-1-yloxy)-3-[(propan-2-yl)amino]propan-2-ol Hydrochloride
196. 2-propanol, 1-(isopropylamino)-3-(1-naphthyloxy)-, Hydrochloride, (+-)-
197. Akos005267141
198. Propranolol Hydrochloride (jp17/usp)
199. (s)-2-fmoc-6-chlorhex-4-ynoic Acid
200. Ks-1097
201. Lp00896
202. Nc00687
203. Nsc-758950
204. Propranolol Hydrochloride [jan]
205. 2-propanol, 1-((1-methylethyl)amino)-3-(1-naphthalenyloxy)-, Hydrochloride, (+-)-
206. Propranolol Hydrochloride [hsdb]
207. Propranolol Hydrochloride [usan]
208. Ncgc00024690-04
209. Ncgc00091932-01
210. Ncgc00094212-01
211. Ncgc00094212-02
212. Ncgc00094212-03
213. Ncgc00094212-04
214. Ncgc00094212-05
215. Ncgc00256554-01
216. Ncgc00259435-01
217. Ncgc00261581-01
218. Propranolol Hydrochloride [mart.]
219. Propranolol Hydrochloride [vandf]
220. Propranolol Hydrochloride [usp-rs]
221. Propranolol Hydrochloride [who-dd]
222. Propranolol Hydrochloride [who-ip]
223. Db-018092
224. Eu-0100896
225. Ft-0603377
226. Ft-0635178
227. Ft-0636788
228. P0995
229. Sw219414-1
230. Wln: L66j Bo1yq1my1 & 1 & Gh
231. Bim-0050871.0001
232. D00483
233. H10708
234. P 0884
235. Propranolol Hydrochloride [ep Monograph]
236. Propranolol Hydrochloride [orange Book]
237. Propranolol Hydrochloride [usp Monograph]
238. Propranolol Hydrochloride 100 Microg/ml In Water
239. Propranololi Hydrochloridum [who-ip Latin]
240. ( Inverted Question Mark)-propranolol Hydrochloride
241. Q-201632
242. Sr-01000075285-1
243. Sr-01000075285-3
244. W-109142
245. Q27108095
246. Z90121066
247. (+/-)-propranolol Hydrochloride, >=99% (tlc), Powder
248. (+/-)-propranolol, Hydrochloride - Cas 3506-09-0
249. (+/-)-propranolol Hydrochloride, Analytical Reference Material
250. 1-(isopropylamino)-3-(1-napthyloxy)-2-propanol Hydrochloride
251. Propranolol Hydrochloride 1.0 Mg/ml In Methanol (as Free Base)
252. (+/-)-[2-hydroxy-3-(naphthyloxy)propyl]isopropylammonium Chloride
253. 1-(1-naphthyloxy)-2-hydroxy-3-(isopropylamino)propane Hydrochloride
254. 1-(1-naphthyloxy)-2-hydroxy-3-isopropylaminopropanehydrochloride
255. 1-(alpha-naphthoxy)-3-(iso-propylamino)-2-propanol Hydrochloride
256. 1-(alpha-naphthoxy)-3-(isopropylamino)-2-propanol Hydrochloride
257. 1-(isopropylamino)-3-(.alpha.-naphthoxy)-2-propanol Hydrochloride
258. Propranolol Hydrochloride, European Pharmacopoeia (ep) Reference Standard
259. ( Inverted Question Mark)-1-(isopropylamino)-3-(1-naphthyloxy)-2-propanol Hydrochloride
260. (rs)-1-[(1-methylethyl)amino]-3-(1-naphthalenyloxy)-2-propanol Hydrochloride
261. (rs)-1-[(1-methylethyl)amino]-3-(1-naphthalenyloxy)-2-propanolhydrochloride
262. Propranolol Hydrochloride, Pharmaceutical Secondary Standard; Certified Reference Material
263. Propranolol Hydrochloride, United States Pharmacopeia (usp) Reference Standard
264. 2-propanol, 1-((1-methylethyl)amino)-3-(1-naphthalenyloxy)-, Hydrochloride, (+/-)-
265. Propranolol Hydrochloride For Performance Test, European Pharmacopoeia (ep) Reference Standard
266. Propranolol Hydrochloride Solution, 1.0 Mg/ml In Methanol (as Free Base), Ampule Of 1 Ml, Certified Reference Material
| Molecular Weight | 295.80 g/mol |
|---|---|
| Molecular Formula | C16H22ClNO2 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 6 |
| Exact Mass | 295.1339066 g/mol |
| Monoisotopic Mass | 295.1339066 g/mol |
| Topological Polar Surface Area | 41.5 Ų |
| Heavy Atom Count | 20 |
| Formal Charge | 0 |
| Complexity | 257 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 10 | |
|---|---|
| Drug Name | Inderal |
| PubMed Health | Propranolol (By mouth) |
| Drug Classes | Antianginal, Antiarrhythmic, Group II, Antihypertensive, Antimigraine, Cardiovascular Agent |
| Drug Label | Inderal (propranolol hydrochloride) is a synthetic beta-adrenergic receptor-blocking agent chemically described as 2-Propanol, 1-[(1-methylethyl)amino]-3-(1-naphthalenyloxy)-, hydrochloride,()-. Its molecular and structural formulae are: C16H21NO... |
| Active Ingredient | Propranolol hydrochloride |
| Dosage Form | Tablet |
| Route | oral |
| Strength | 60mg; 10mg; 80mg; 40mg; 20mg |
| Market Status | Prescription |
| Company | Akrimax Pharms |
| 2 of 10 | |
|---|---|
| Drug Name | Inderal la |
| PubMed Health | Somatropin, E coli Derived (Injection) |
| Drug Classes | Endocrine-Metabolic Agent |
| Drug Label | Inderal (propranolol hydrochloride) is a synthetic beta-adrenergic receptor-blocking agent chemically described as 2-Propanol, 1-[(1-methylethyl)amino]-3-(1-naphthalenyloxy)-, hydrochloride,()-. Its molecular and structural formulae are: C16H21NO... |
| Active Ingredient | Propranolol hydrochloride |
| Dosage Form | Capsule, extended release |
| Route | Oral |
| Strength | 160mg; 120mg; 60mg; 80mg |
| Market Status | Prescription |
| Company | Akrimax Pharms |
| 3 of 10 | |
|---|---|
| Drug Name | Innopran xl |
| PubMed Health | Propranolol |
| Drug Classes | Antianginal, Antiarrhythmic, Group II, Antihypertensive, Antimigraine, Cardiovascular Agent |
| Drug Label | INNOPRAN XL (propranolol hydrochloride) is a nonselective, beta-adrenergic receptor-blocking agent for oral administration, available as an extended release product. INNOPRAN XL is available as 80-mg and 120-mg capsules which contain sustained-releas... |
| Active Ingredient | Propranolol hydrochloride |
| Dosage Form | Capsule, extended release |
| Route | Oral |
| Strength | 120mg; 80mg |
| Market Status | Prescription |
| Company | Glaxosmithkline |
| 4 of 10 | |
|---|---|
| Drug Name | Propranolol hydrochloride |
| PubMed Health | Propranolol (Injection) |
| Drug Classes | Antianginal, Antiarrhythmic, Group II, Antihypertensive, Antimigraine, Cardiovascular Agent |
| Drug Label | hydrochloride(Propranolol is a synthetic beta-adrenergic receptor blocking agent chemically described as 2-Propanol, 1-[(1-methylethyl)amino]-3-(1-naphthalenyloxy)-, hydrochloride,()-. Its molecular and structural formulae are:C16H21NO2.HClPropr... |
| Active Ingredient | Propranolol hydrochloride |
| Dosage Form | Tablet; Injectable; Capsule, extended release; Solution |
| Route | Injection; Oral |
| Strength | 1mg/ml; 160mg; 40mg/5ml; 120mg; 60mg; 10mg; 20mg/5ml; 80mg; 40mg; 20mg |
| Market Status | Prescription |
| Company | Vintage Pharms; Upsher Smith; Bedford; Aptalis Pharmatech; Hikma Farmaceutica; Northstar Hlthcare; Sandoz; Roxane; Watson Labs; Glatt Air; Actavis Elizabeth; Baxter Hlthcare; Fresenius Kabi Usa; Ipca Labs; Zydus Pharms Usa; Pliva; Mylan |
| 5 of 10 | |
|---|---|
| Drug Name | Tev-tropin |
| Active Ingredient | Somatropin recombinant |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 5mg/vial |
| Market Status | Prescription |
| Company | Ferring |
| 6 of 10 | |
|---|---|
| Drug Name | Inderal |
| PubMed Health | Propranolol (By mouth) |
| Drug Classes | Antianginal, Antiarrhythmic, Group II, Antihypertensive, Antimigraine, Cardiovascular Agent |
| Drug Label | Inderal (propranolol hydrochloride) is a synthetic beta-adrenergic receptor-blocking agent chemically described as 2-Propanol, 1-[(1-methylethyl)amino]-3-(1-naphthalenyloxy)-, hydrochloride,()-. Its molecular and structural formulae are: C16H21NO... |
| Active Ingredient | Propranolol hydrochloride |
| Dosage Form | Tablet |
| Route | oral |
| Strength | 60mg; 10mg; 80mg; 40mg; 20mg |
| Market Status | Prescription |
| Company | Akrimax Pharms |
| 7 of 10 | |
|---|---|
| Drug Name | Inderal la |
| PubMed Health | Somatropin, E coli Derived (Injection) |
| Drug Classes | Endocrine-Metabolic Agent |
| Drug Label | Inderal (propranolol hydrochloride) is a synthetic beta-adrenergic receptor-blocking agent chemically described as 2-Propanol, 1-[(1-methylethyl)amino]-3-(1-naphthalenyloxy)-, hydrochloride,()-. Its molecular and structural formulae are: C16H21NO... |
| Active Ingredient | Propranolol hydrochloride |
| Dosage Form | Capsule, extended release |
| Route | Oral |
| Strength | 160mg; 120mg; 60mg; 80mg |
| Market Status | Prescription |
| Company | Akrimax Pharms |
| 8 of 10 | |
|---|---|
| Drug Name | Innopran xl |
| PubMed Health | Propranolol |
| Drug Classes | Antianginal, Antiarrhythmic, Group II, Antihypertensive, Antimigraine, Cardiovascular Agent |
| Drug Label | INNOPRAN XL (propranolol hydrochloride) is a nonselective, beta-adrenergic receptor-blocking agent for oral administration, available as an extended release product. INNOPRAN XL is available as 80-mg and 120-mg capsules which contain sustained-releas... |
| Active Ingredient | Propranolol hydrochloride |
| Dosage Form | Capsule, extended release |
| Route | Oral |
| Strength | 120mg; 80mg |
| Market Status | Prescription |
| Company | Glaxosmithkline |
| 9 of 10 | |
|---|---|
| Drug Name | Propranolol hydrochloride |
| PubMed Health | Propranolol (Injection) |
| Drug Classes | Antianginal, Antiarrhythmic, Group II, Antihypertensive, Antimigraine, Cardiovascular Agent |
| Drug Label | hydrochloride(Propranolol is a synthetic beta-adrenergic receptor blocking agent chemically described as 2-Propanol, 1-[(1-methylethyl)amino]-3-(1-naphthalenyloxy)-, hydrochloride,()-. Its molecular and structural formulae are:C16H21NO2.HClPropr... |
| Active Ingredient | Propranolol hydrochloride |
| Dosage Form | Tablet; Injectable; Capsule, extended release; Solution |
| Route | Injection; Oral |
| Strength | 1mg/ml; 160mg; 40mg/5ml; 120mg; 60mg; 10mg; 20mg/5ml; 80mg; 40mg; 20mg |
| Market Status | Prescription |
| Company | Vintage Pharms; Upsher Smith; Bedford; Aptalis Pharmatech; Hikma Farmaceutica; Northstar Hlthcare; Sandoz; Roxane; Watson Labs; Glatt Air; Actavis Elizabeth; Baxter Hlthcare; Fresenius Kabi Usa; Ipca Labs; Zydus Pharms Usa; Pliva; Mylan |
| 10 of 10 | |
|---|---|
| Drug Name | Tev-tropin |
| Active Ingredient | Somatropin recombinant |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 5mg/vial |
| Market Status | Prescription |
| Company | Ferring |
Adrenergic beta-Antagonists; Anti-Anxiety Agents; Anti-Arrhythmia Agents; Antihypertensive Agents; Sympatholytics; Vasodilator Agents
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
/SRP: Former use/: Propranolol has proven to be effective in numerous cases in which digitalis, with or without quinidine and/or procainamide, failed to reduce ventricular rate, and in cases of paroxysmal atrial tachycardia attributed to digitalis toxicity.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 700
Propranolol is also used in hypertrophic obstructive cardiomyopathies. In these conditions forceful contraction of myocardium along a ventricular outflow tract can greatly increase outflow resistance, particularly during exercise. .../It/ is sometimes useful in management of tachycardia and arrhythmias in patient with pheochromocytoma.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 552
Medication (Vet): ...Atropine in conjunction with propranolol /was found/ to be useful in treatment of oleander poisoning.
Clarke, E.G., and M. L. Clarke. Veterinary Toxicology. Baltimore, Maryland: The Williams and Wilkins Company, 1975., p. 275
For more Therapeutic Uses (Complete) data for PROPRANOLOL HYDROCHLORIDE (25 total), please visit the HSDB record page.
.../Propranolol/ is relatively contraindicated in ...hay fever, cardiogenic shock, congestive heart failure, right ventricular failure secondary to pulmonary hypertension, and when myocardial depressant anesthetics, tricyclic antidepressants, or oral hypoglycemics are used.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 835
Propranolol (1% solution) used as eye-drops .../per 1 report/, caused intense pain lasting as long as 15 min and induced hyperemia and slight miosis, but according to others these eye-drops have been well tolerated by most patients in use up to 4 times/day for 3-4 months, causing burning sensations and conjunctival hyperemia in only 8/47 eyes.
Grant, W.M. Toxicology of the Eye. 3rd ed. Springfield, IL: Charles C. Thomas Publisher, 1986., p. 768
Contraindicated in patients with cardiogenic shock, sinus bradycardia and greater than first degree block, bronchial asthma, and congestive heart failure. Adverse reactions include weakness, light headedness, depression, bradycardia, paresthesia of hands, arterial insufficiency (e.g., Raynaud type), nausea, and diarrhea. Use is best avoided in patients with bronchospastic diseases and therapy in diabetic patients must be closely monitored.
Hussar, D.A. (ed.). Modell's Drugs in Current Use and New Drugs. 38th ed. New York, NY: Springer Publishing Co., 1992., p. 140
After sudden cessation of propranolol therapy in some patients treated for angina, increased frequency, duration, and severity of angina episodes have occurred, often within 24 hr. These episodes are unstable and are not relieved by nitroglycerin. Acute and sometimes fatal myocardial infarction and sudden death have also occurred after abrupt withdrawal of propranolol therapy in some patients treated for angina. In hypertensive patients, sudden cessation of propranolol has produced a syndrome similar to florid thyrotoxicosis, characterized by tenseness, anxiety, tachycardia, and excessive perspiration; these symptoms occurred within one week of cessation of the drug and were relieved by reinstituting propranolol therapy.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 1782
For more Drug Warnings (Complete) data for PROPRANOLOL HYDROCHLORIDE (31 total), please visit the HSDB record page.
Hemangiol is indicated in the treatment of proliferating infantile haemangioma requiring systemic therapy:
- Life- or function-threatening haemangioma,
- Ulcerated haemangioma with pain and/or lack of response to simple wound care measures,
- Haemangioma with a risk of permanent scars or disfigurement.
It is to be initiated in infants aged 5 weeks to 5 months.
Adrenergic beta-Antagonists
Drugs that bind to but do not activate beta-adrenergic receptors thereby blocking the actions of beta-adrenergic agonists. Adrenergic beta-antagonists are used for treatment of hypertension, cardiac arrhythmias, angina pectoris, glaucoma, migraine headaches, and anxiety. (See all compounds classified as Adrenergic beta-Antagonists.)
Antihypertensive Agents
Drugs used in the treatment of acute or chronic vascular HYPERTENSION regardless of pharmacological mechanism. Among the antihypertensive agents are DIURETICS; (especially DIURETICS, THIAZIDE); ADRENERGIC BETA-ANTAGONISTS; ADRENERGIC ALPHA-ANTAGONISTS; ANGIOTENSIN-CONVERTING ENZYME INHIBITORS; CALCIUM CHANNEL BLOCKERS; GANGLIONIC BLOCKERS; and VASODILATOR AGENTS. (See all compounds classified as Antihypertensive Agents.)
Vasodilator Agents
Drugs used to cause dilation of the blood vessels. (See all compounds classified as Vasodilator Agents.)
Anti-Arrhythmia Agents
Agents used for the treatment or prevention of cardiac arrhythmias. They may affect the polarization-repolarization phase of the action potential, its excitability or refractoriness, or impulse conduction or membrane responsiveness within cardiac fibers. Anti-arrhythmia agents are often classed into four main groups according to their mechanism of action: sodium channel blockade, beta-adrenergic blockade, repolarization prolongation, or calcium channel blockade. (See all compounds classified as Anti-Arrhythmia Agents.)
C07AA05
Studies in man and experimental animals indicate that rapid hepatic clearance is responsible for appearance of only trace amount of unmetabolized propranolol in blood after small oral doses. With larger doses, blood levels are linearly related to dose, suggesting saturation of hepatic metabolic system.
The Chemical Society. Foreign Compound Metabolism in Mammals Volume 3. London: The Chemical Society, 1975., p. 144
Propranolol is almost completely absorbed from the GI tract; however, plasma concentrations attained are quite variable among individuals. There is no difference in the rate of absorption of the 2 isomers of propranolol. Propranolol appears in the plasma within 30 min, and peak plasma concentrations are reached about 60-90 min after oral administration of the conventional tablets. The time when peak plasma concentrations are reached may be delayed, but concentrations are not necessarily lowered, when the drug is administered with food. Oral bioavailability of the drug may be increased in children with Down's syndrome; higher than expected plasma propranolol concentrations have been observed in such children. Bioavailability of a single 40-mg oral dose of propranolol hydrochloride as a conventional tablet or oral solution reportedly is equivalent in adults.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 1785-6
Propranolol hydrochloride is slowly absorbed following administration of the drug as extended release capsules, and peak blood concentrations are reached about 6 hr after administration. When measured at steady state over a 24 hr period, the area under the plasma concentration time curve for the extended release capsules is about 60-65% of the plasma concentration time curve for a comparable divided daily dose of the conventional tablets. The lower plasma concentration time curve is probably caused by the slower rate of absorption of the drug from the extended release capsules with resultant greater hepatic metabolism. After administration of a single dose of propranolol as the extended release capsules, blood concentrations are fairly constant for about 12 hr and then decline exponentially during the following 12 hr.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 1785-6
Following iv administration of propranolol, the onset of action is almost immediate. Animal studies indicate that propranolol is rapidly absorbed after im administration.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 1786
For more Absorption, Distribution and Excretion (Complete) data for PROPRANOLOL HYDROCHLORIDE (12 total), please visit the HSDB record page.
Besides ... 4-hydroxypropranolol and naphthoxylacetic acid, 6 new urinary metabolites have... been identified... /which are/ n-deisopropylpropranolol; 1-(alpha-naphthoxy)-2,3-propyleneglycol; ring hydroxylated 1-(alpha-naphthoxy)-2,3-propyleneglycol; alpha-naphthoxyacetic acid; alpha-naphthol and 1,4-dihydroxynaphthalene.
The Chemical Society. Foreign Compound Metabolism in Mammals Volume 3. London: The Chemical Society, 1975., p. 215
Isopropylamine and hexadeuteriated isopropylamine have been identified as urinary metabolites of propranolol and hexadeuteriated propranolol, respectively; this is believed to be 1st recorded example of single-step oxidative deamination of n-isopropylamine compound.
The Chemical Society. Foreign Compound Metabolism in Mammals Volume 3. London: The Chemical Society, 1975., p. 217
During initial oral therapy (but not during iv or chronic oral therapy), an active metabolite, 4-hydroxypropranolol, is formed. 4-Hydroxypropranolol has about the same beta-adrenergic blocking potency as does propranolol and may be present in plasma in amounts about equal to propranolol. This metabolite is eliminated more rapidly than propranolol and is virtually absent from the plasma 6 hr after oral administration of the drug. Results of one study indicate that after iv administration or chronic oral administration of propranolol, 4-hydroxypropranolol is not formed to a substantial extent, and beta-adrenergic blocking activity is more closely reflected by propranolol concentrations. Individual variations in ability to hydroxylate propranolol to the active metabolite may also exist. In addition, some other metabolites of propranolol may possess antiarrhythmic activity without beta-adrenergic blocking activity.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 1786
Propranolol is almost completely metabolized in the liver and at least 8 metabolites have been identified in urine. Only 1-4% of an oral or iv dose of the drug appears in feces as unchanged drug and metabolites.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 1786
When usual therapeutic doses of propranolol are administered chronically, the half-life ranges from 3.4-6 hr. Single dose studies generally have shown a shorter half-life of 2-3 hr.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 1786
Propranolol is a nonselective beta-adrenergic blocking agent. Propranolol inhibits response to adrenergic stimuli by competitively blocking, beta-adrenergic receptors within the myocardium and within bronchial and vascular smooth muscle. Only the l-isomer of propranolol has substantial beta-adrenergic blocking activity. Propranolol has no intrinsic sympathomimetic activity.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 1785
Through its myocardial, beta-adrenergic blocking action, propranolol decreases heart rate and prevents exercise induced increases in heart rate, decreases myocardial contractility, decreases cardiac output, increases systolic ejection time, and increases cardiac volume. The drug also decreases conduction velocity through the sinoatrial and atrioventricular nodes and decreases myocardial automaticity via beta-adrenergic blockade. At blood concentrations greater than those required for beta-adrenergic blockade, propranolol has a membrane stabilizing effect on the heart which is similar to that of quinidine. The clinical importance of this effect is not clear, but it appears to be less important than its beta-adrenergic blocking activity.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 1785
beta-Adrenergic blockade may also incr peripheral resistance initially, but peripheral resistance tends to decr after chronic admin of the drug as a result of unopposed alpha-adrenergic vasoconstriction. The cardiac effects of, beta-adrenergic blockade cause an incr in sodium reabsorption because of alterations in renal hemodynamics; renal blood flow and glomerular filtration rate generally decr during chronic therapy. Plasma volume may incr if dietary sodium is not restricted. Hepatic blood flow is decreased.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 1785
The precise mechanism of propranolol's hypotensive effect has not been determined. It has been postulated that beta-adrenergic blocking agents reduce blood pressure by blocking peripheral (especially cardiac) adrenergic receptors (decreasing cardiac output), by decreasing sympathetic outflow from the CNS, and/or by suppressing renin release. In patients with high concentrations of circulating renin, low doses of the drug are associated with a fall in both blood pressure and in plasma renin concentrations, probably because of acute peripheral beta-adrenergic blockade. With higher doses of propranolol, the hypotensive effect is probably unrelated to plasma renin activity and may be caused by a delayed centrally mediated reduction of adrenergic outflow. However, there appears to be some overlap between these mechanisms, and both mechanisms seem to be operative with usual therapeutic doses. Propranolol decreases blood pressure in both the supine and standing positions.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 1785
Through its beta-adrenergic blocking action in other body systems, propranolol increases airway resistance (especially in asthmatic patients), inhibits glycogenolysis in the skeletal and cardiac muscles, blocks the release of free fatty acids and insulin by adrenergic stimulation, and increases the number of circulating eosinophils. Propranolol increases uterine activity, more in the nonpregnant than in the pregnant uterus.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 1785
 LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
About the Company : LGM Pharma is a global leader in sourcing hard-to-find APIs and intermediates for pharmaceutical and biotech industries. LGM also operates as a full-service CDMO, offering formulat...
 Nuray is an expert in the synthesis of Niche novel APIs, the first to launch Generics, NCEs, Advanced Intermediates // USFDA certified.
Nuray is an expert in the synthesis of Niche novel APIs, the first to launch Generics, NCEs, Advanced Intermediates // USFDA certified.
About the Company : Nuray Chemicals Pvt Ltd, established in 2012 near Chennai, is an API manufacturer for highly regulated markets. Its state-of-the-art R&D facility specializes in synthesizing NCEs, ...
About the Company : Jai Radhe Sales, founded in 1999, is a global distributor specializing in high-quality pharmaceutical ingredients from India. It offers complete sourcing solutions, technical and r...
 Tenatra connects Indian manufacturers with global buyers through active partners in Germany, Switzerland, Belgium, Spain & Turkey.
Tenatra connects Indian manufacturers with global buyers through active partners in Germany, Switzerland, Belgium, Spain & Turkey.
About the Company : Tenatra International was established as a proprietorship firm in 1999. It got off to a very good start, supporting clients in the United States, Mexico and Europe. As business opp...
 Delivering quality and niche Active Pharmaceutical Ingredients across the global from our USFDA-approved facility.
Delivering quality and niche Active Pharmaceutical Ingredients across the global from our USFDA-approved facility.
About the Company : Mankind Pharma was established in 1986 and began operating as a fully integrated pharmaceutical company in 1995. The company offers a broad portfolio across multiple therapeutic ca...
 Cosma S.p.A., part of CFM Group with AMSA & Clarochem, provides global pharma & veterinary health with 300+ tons of FDA-approved APIs.
Cosma S.p.A., part of CFM Group with AMSA & Clarochem, provides global pharma & veterinary health with 300+ tons of FDA-approved APIs.
About the Company : Cosma S.p.A., part of the CFM Group with AMSA and Clarochem Ireland, is the group’s largest manufacturing site in Bergamo, Italy. With 130,000L glass-lined reaction volume and ov...
About the Company : Darou Pakhsh Pharma Chem (DPPC Co.) as one of the qualified and leading producers of active pharmaceutical ingredients in the Iranian pharmaceutical industry started production sin...

About the Company : Globe Quimica S.A. is a major Brazilian API producer, GMP certificated by ANVISA, manufactures more than 20 different API's such as Antiretrovirals, Anxiolytic, Antidepressant, Ant...

About the Company : Livmore Life Sciences Pvt. Ltd., established in 2016, is a pharmaceutical company dedicated to the development, optimization, and manufacturing of high-quality Active Pharmaceutica...

About the Company : Osmopharm S.A. is a GMP approved pharmaceutical company located in Switzerland and specialized in development and production of modified release solid oral form drugs under contrac...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Details:
Propranolol is a small molecule drug, which is currently being evaluated in Phase IV clinical studies for the treatment of idiopathic ventricular fibrillation.
Lead Product(s): Propranolol Hydrochloride,Nadolol,Atenolol,Bisoprolol Fumarate,Metoprolol Tartrate
Therapeutic Area: Cardiology/Vascular Diseases Brand Name: Undisclosed
Study Phase: Phase IVProduct Type: Miscellaneous
Sponsor: Per Henriksen Foundation | Danish Heart Foundation | RH research funds | Bo Gregers Winkel
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable February 12, 2026

Lead Product(s) : Propranolol Hydrochloride,Nadolol,Atenolol,Bisoprolol Fumarate,Metoprolol Tartrate
Therapeutic Area : Cardiology/Vascular Diseases
Highest Development Status : Phase IV
Partner/Sponsor/Collaborator : Per Henriksen Foundation | Danish Heart Foundation | RH research funds | Bo Gregers Winkel
Deal Size : Inapplicable
Deal Type : Inapplicable
MEdical Treatment in Idiopathic Ventricular Fibrillation Patients
Details : Propranolol is a small molecule drug, which is currently being evaluated in Phase IV clinical studies for the treatment of idiopathic ventricular fibrillation.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
February 12, 2026

Details:
Propranolol is a small molecule drug candidate, which is currently being evaluated in Phase I clinical studies for the treatment of Misophonia.
Lead Product(s): Propranolol Hydrochloride,Inapplicable
Therapeutic Area: Neurology Brand Name: Undisclosed
Study Phase: Phase IProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable December 09, 2025

Lead Product(s) : Propranolol Hydrochloride,Inapplicable
Therapeutic Area : Neurology
Highest Development Status : Phase I
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : Propranolol is a small molecule drug candidate, which is currently being evaluated in Phase I clinical studies for the treatment of Misophonia.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
December 09, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Atogepant is a Other Small Molecule drug candidate, which is currently being evaluated in phase IV clinical studies for the treatment of Migraine Disorders.
Lead Product(s): Atogepant,Propranolol Hydrochloride,Topiramate
Therapeutic Area: Neurology Brand Name: Undisclosed
Study Phase: Phase IVProduct Type: Miscellaneous
Sponsor: University of Iowa | Patient-Centered Outcomes Research Institute | AbbVie Inc
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable May 14, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Atogepant,Propranolol Hydrochloride,Topiramate
Therapeutic Area : Neurology
Highest Development Status : Phase IV
Partner/Sponsor/Collaborator : University of Iowa | Patient-Centered Outcomes Research Institute | AbbVie Inc
Deal Size : Inapplicable
Deal Type : Inapplicable
Comparative Effectiveness of Migraine Preventive Medications: The APT Comparison Study
Details : Atogepant is a Other Small Molecule drug candidate, which is currently being evaluated in phase IV clinical studies for the treatment of Migraine Disorders.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
May 14, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
GPC-100 (burixafor) is a highly selective small molecule antagonist of CXCR4, being investigated with propranolol and G-CSF in multiple myeloma patients undergoing autologous transplant.
Lead Product(s): Burixafor Hydrobromide,GM CSF,Propranolol Hydrochloride,G-CSF
Therapeutic Area: Oncology Brand Name: Undisclosed
Study Phase: Phase IIProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable May 05, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Burixafor Hydrobromide,GM CSF,Propranolol Hydrochloride,G-CSF
Therapeutic Area : Oncology
Highest Development Status : Phase II
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Exicure Completes Enrollment for GPC-100 Phase 2 Myeloma Transplant Study
Details : GPC-100 (burixafor) is a highly selective small molecule antagonist of CXCR4, being investigated with propranolol and G-CSF in multiple myeloma patients undergoing autologous transplant.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
May 05, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
GPC-100 (Burixafor HBr) is a novel small molecule CXCR4 antagonist, being investigated in patients with multiple myeloma.
Lead Product(s): Burixafor Hydrobromide,Propranolol Hydrochloride,G-CSF
Therapeutic Area: Oncology Brand Name: Undisclosed
Study Phase: Phase IIProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable April 14, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Burixafor Hydrobromide,Propranolol Hydrochloride,G-CSF
Therapeutic Area : Oncology
Highest Development Status : Phase II
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Exicure Nears Completion of Phase 2 Study of GPC-100 in Multiple Myeloma
Details : GPC-100 (Burixafor HBr) is a novel small molecule CXCR4 antagonist, being investigated in patients with multiple myeloma.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
April 14, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Through the acquisition, Exicure will leverage GPCR USA pipeline, which include GPC-100 (burixafor+propranolol) with G-CSF for the treatment of Multiple Myeloma.
Lead Product(s): Burixafor Hydrobromide,G-CSF,Propranolol Hydrochloride
Therapeutic Area: Oncology Brand Name: Undisclosed
Study Phase: Phase IIProduct Type: Miscellaneous
Sponsor: Exicure
Deal Size: Undisclosed Upfront Cash: Undisclosed
Deal Type: Acquisition January 22, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Burixafor Hydrobromide,G-CSF,Propranolol Hydrochloride
Therapeutic Area : Oncology
Highest Development Status : Phase II
Partner/Sponsor/Collaborator : Exicure
Deal Size : Undisclosed
Deal Type : Acquisition
Exicure Announces Purchase Agreement with GPCR Therapeutics
Details : Through the acquisition, Exicure will leverage GPCR USA pipeline, which include GPC-100 (burixafor+propranolol) with G-CSF for the treatment of Multiple Myeloma.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Undisclosed
January 22, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
The partnership aims to advance the clinical development of the company's mid-stage product GPC-201 (GPC-100+Propanalol) for treating patients suffering from Multiple Myeloma.
Lead Product(s): GPC-100,Propranolol Hydrochloride
Therapeutic Area: Oncology Brand Name: Undisclosed
Study Phase: Phase IIProduct Type: Miscellaneous
Sponsor: GPCR Therapeutics
Deal Size: Undisclosed Upfront Cash: Undisclosed
Deal Type: Partnership December 26, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : GPC-100,Propranolol Hydrochloride
Therapeutic Area : Oncology
Highest Development Status : Phase II
Partner/Sponsor/Collaborator : GPCR Therapeutics
Deal Size : Undisclosed
Deal Type : Partnership
Exicure Partners with GPCR Therapeutics to Fuel New Growth in Biotech
Details : The partnership aims to advance the clinical development of the company's mid-stage product GPC-201 (GPC-100+Propanalol) for treating patients suffering from Multiple Myeloma.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Undisclosed
December 26, 2024

Details:
Propranolol is a Other Small Molecule drug candidate, which is currently being evaluated in phase I clinical studies for the treatment of Brain Injuries, Traumatic.
Lead Product(s): Propranolol Hydrochloride,Inapplicable
Therapeutic Area: Trauma (Emergency, Injury, Surgery) Brand Name: Undisclosed
Study Phase: Phase IProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable February 15, 2024

Lead Product(s) : Propranolol Hydrochloride,Inapplicable
Therapeutic Area : Trauma (Emergency, Injury, Surgery)
Highest Development Status : Phase I
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Propranolol for the Treatment of Traumatic Brain Injury
Details : Propranolol is a Other Small Molecule drug candidate, which is currently being evaluated in phase I clinical studies for the treatment of Brain Injuries, Traumatic.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
February 15, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Inderal-Generic (propranolol) is a β-adrenoceptors antagonist. It is now approved for the treatment of hypertension, angina pectoris, migraine, hypertrophic subaortic stenosis.
Lead Product(s): Propranolol Hydrochloride,Inapplicable
Therapeutic Area: Cardiology/Vascular Diseases Brand Name: Inderal-Generic
Study Phase: Approved FDFProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable January 14, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Propranolol Hydrochloride,Inapplicable
Therapeutic Area : Cardiology/Vascular Diseases
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Lupin Gets USFDA Nod for Generic Hypertension Drug
Details : Inderal-Generic (propranolol) is a β-adrenoceptors antagonist. It is now approved for the treatment of hypertension, angina pectoris, migraine, hypertrophic subaortic stenosis.
Product Name : Inderal-Generic
Product Type : Miscellaneous
Upfront Cash : Inapplicable
January 14, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Propranolol hydrochloride tablet is a synthetic beta-adrenergic receptor blocking agent, which is indicated for the treatment of the management of hypertension.
Lead Product(s): Propranolol Hydrochloride,Inapplicable
Therapeutic Area: Cardiology/Vascular Diseases Brand Name: Inderal-Generic
Study Phase: Approved FDFProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable August 29, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Propranolol Hydrochloride,Inapplicable
Therapeutic Area : Cardiology/Vascular Diseases
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Lupin Launches Propranolol Long-Acting Capsules to Improve Heart Health in Canada
Details : Propranolol hydrochloride tablet is a synthetic beta-adrenergic receptor blocking agent, which is indicated for the treatment of the management of hypertension.
Product Name : Inderal-Generic
Product Type : Miscellaneous
Upfront Cash : Inapplicable
August 29, 2023

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
Regulatory Info : Allowed
Registration Country : Switzerland
Brand Name : Propranolol Zentiva
Dosage Form : Film Coated Tablet
Dosage Strength : 10mg
Packaging :
Approval Date : 10/10/1985
Application Number : 47025
Regulatory Info : Allowed
Registration Country : Switzerland
 Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
Regulatory Info : Allowed
Registration Country : Switzerland
Brand Name : Propranolol Zentiva
Dosage Form : Film Coated Tablet
Dosage Strength : 40mg
Packaging :
Approval Date : 10/10/1985
Application Number : 47025
Regulatory Info : Allowed
Registration Country : Switzerland
 Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
Regulatory Info : Allowed
Registration Country : Switzerland
Brand Name : Propranolol Zentiva
Dosage Form : Film Coated Tablet
Dosage Strength : 80mg
Packaging :
Approval Date : 10/10/1985
Application Number : 47025
Regulatory Info : Allowed
Registration Country : Switzerland
 Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
Regulatory Info : Allowed
Registration Country : Switzerland
Brand Name : Propranolol retard Zentiva
Dosage Form : Capsule
Dosage Strength : 160mg
Packaging :
Approval Date : 12/12/1985
Application Number : 47551
Regulatory Info : Allowed
Registration Country : Switzerland
Regulatory Info :
Registration Country : India
Brand Name : Propranolol Hydrochloride
Dosage Form : Pellets
Dosage Strength : 47%
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India
 Olpha, a JSC AB City subsidiary, is a leading Baltic firm with 50 years of experience in medicines & chemical pharmaceuticals.
Olpha, a JSC AB City subsidiary, is a leading Baltic firm with 50 years of experience in medicines & chemical pharmaceuticals.
Regulatory Info :
Registration Country : Latvia
Brand Name : Anaprilīn
Dosage Form : Tablet
Dosage Strength : 10MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Latvia
Regulatory Info :
Registration Country : India
Brand Name : Propranolol Hydrochloride
Dosage Form : Tablet
Dosage Strength : 90MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : DISCN
Registration Country : USA
Brand Name : INDERAL
Dosage Form : TABLET;ORAL
Dosage Strength : 10MG **Federal Register determination that product was not discontinued or withdrawn for safety or effectiveness reasons**
Packaging :
Approval Date : 1982-01-01
Application Number : 16418
Regulatory Info : DISCN
Registration Country : USA

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : DISCN
Registration Country : USA
Brand Name : INDERAL
Dosage Form : TABLET;ORAL
Dosage Strength : 40MG **Federal Register determination that product was not discontinued or withdrawn for safety or effectiveness reasons**
Packaging :
Approval Date : 1982-01-01
Application Number : 16418
Regulatory Info : DISCN
Registration Country : USA

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : DISCN
Registration Country : USA
Brand Name : INDERAL
Dosage Form : TABLET;ORAL
Dosage Strength : 20MG **Federal Register determination that product was not discontinued or withdrawn for safety or effectiveness reasons**
Packaging :
Approval Date : 1982-01-01
Application Number : 16418
Regulatory Info : DISCN
Registration Country : USA

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Regulatory Info :
Registration Country : India
Brand Name : Propranolol Hydrochlor...
Dosage Form : Pellets
Dosage Strength : 47%
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India
Packaging :
Regulatory Info :
Dosage : Pellets
Dosage Strength : 47%
Brand Name : Propranolol Hydrochlor...
Approval Date :
Application Number :
Registration Country : India
 Olpha, a JSC AB City subsidiary, is a leading Baltic firm with 50 years of experience in medicines & chemical pharmaceuticals.
Olpha, a JSC AB City subsidiary, is a leading Baltic firm with 50 years of experience in medicines & chemical pharmaceuticals.
Regulatory Info :
Registration Country : Latvia
Brand Name : Anaprilīn
Dosage Form : Tablet
Dosage Strength : 10MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Latvia
 Olpha, a JSC AB City subsidiary, is a leading Baltic firm with 50 years of experience in medicines & chemical pharmaceuticals.
Olpha, a JSC AB City subsidiary, is a leading Baltic firm with 50 years of experience in medicines & chemical pharmaceuticals.
Packaging :
Regulatory Info :
Dosage : Tablet
Dosage Strength : 10MG
Brand Name : Anaprilīn
Approval Date :
Application Number :
Registration Country : Latvia
Regulatory Info :
Registration Country : India
Brand Name : Propranolol Hydrochlor...
Dosage Form : Tablet
Dosage Strength : 90MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India
Packaging :
Regulatory Info :
Dosage : Tablet
Dosage Strength : 90MG
Brand Name : Propranolol Hydrochlor...
Approval Date :
Application Number :
Registration Country : India
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : ER Tablet
Dosage Strength : 120MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info :
Dosage : ER Tablet
Dosage Strength : 120MG
Brand Name :
Approval Date :
Application Number :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Tablet
Dosage Strength : 10MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info :
Dosage : Tablet
Dosage Strength : 10MG
Brand Name :
Approval Date :
Application Number :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : India
Brand Name : INDERAL LA
Dosage Form : Extended Release Capsu...
Dosage Strength : 160MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info :
Dosage : Extended Release Capsu...
Dosage Strength : 160MG
Brand Name : INDERAL LA
Approval Date :
Application Number :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generics
Registration Country : Costa Rica
Brand Name : propranolol CHEMO®
Dosage Form : COATED TABLET
Dosage Strength : 10MG
Packaging :
Approval Date :
Application Number :
Regulatory Info : Generics
Registration Country : Costa Rica

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info : Generics
Dosage : COATED TABLET
Dosage Strength : 10MG
Brand Name : propranolol CHEMO®
Approval Date :
Application Number :
Registration Country : Costa Rica

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Tablet
Dosage Strength : 10MG
Packaging : 3 x 10 Tablets Blister pack
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging : 3 x 10 Tablets Blister pack
Regulatory Info :
Dosage : Tablet
Dosage Strength : 10MG
Brand Name :
Approval Date :
Application Number :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Coated SR Pellets
Dosage Strength : 21%/W/W
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info :
Dosage : Coated SR Pellets
Dosage Strength : 21%/W/W
Brand Name :
Approval Date :
Application Number :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Turkey
Brand Name :
Dosage Form : Film Coated Tablet
Dosage Strength : 10MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Turkey

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info :
Dosage : Film Coated Tablet
Dosage Strength : 10MG
Brand Name :
Approval Date :
Application Number :
Registration Country : Turkey

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Dosage Form : Powder
Grade : Oral
Dosage Form : Capsule
Grade : Oral
Dosage Form : Capsule
Grade : Oral
Dosage Form : Capsule
Grade : Oral
Dosage Form : Capsule
Grade : Oral
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Dosage Form : Tablet
Grade : Oral
Application : Controlled & Modified Release
Excipient Details : ACTILLETS are microcrystalline cellulose spheres developed using advanced drug delivery technology to enable effective loading & coating of particles.
Pharmacopoeia Ref : NA
Technical Specs : Not Available
Ingredient(s) : Microcrystalline Cellulose Excipients
Dosage Form : Tablet
Grade : Oral
Dosage Form : Tablet
Grade : Oral
Dosage Form : Tablet
Grade : Oral
Application : Taste Masking
Excipient Details : CS90 is a directly compressible calcium carbonate with starch used for chewable tablets due to its smooth mouthfeel and creamy texture.
Pharmacopoeia Ref : NA
Technical Specs : PSD D50: 150-175 µm, Tapped Density: 0.85
Ingredient(s) : Calcium Carbonate Excipient
Dosage Form : Tablet
Grade : Oral
Application : Taste Masking
Excipient Details : MS90 is a directly compressible magnesium hydroxide with starch used for chewable tablets due to its smooth mouthfeel and creamy texture.
Pharmacopoeia Ref : NA
Technical Specs : PSD D50: 150-170 µm, Tapped Density: 0.80
Ingredient(s) : Magnesium Hydroxide Excipient
Dosage Form : Softgels
Grade : Oral
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Dosage Form : Capsule
Grade : Not Available
Application : Disintegrants & Superdisintegrants
Excipient Details : They are references multipurpose superdisintegrants, well-known and widely used in the pharmaceutical industry.
Pharmacopoeia Ref : EP/USP/JPE
Technical Specs : Not Available
Ingredient(s) : Sodium Starch Glycolate
Dosage Form : Softgel Capsule
Grade : Not Available
Application : Soft Gelatin
Dosage Form : Tablet
Grade : Not Available
Application : Fillers, Diluents & Binders
Excipient Details : It is a natural pregelatinized maize starch that has been specially developed as a binder for wet granulation.
Dosage Form : Tablet
Grade : Not Available
Application : Disintegrants & Superdisintegrants
Excipient Details : Used as disintegrants, fillers and binders (once cooked) in nutraceutical and pharmaceutical dosage forms.
Dosage Form : Tablet
Grade : Not Available
Application : Fillers, Diluents & Binders
Excipient Details : Helps to manufacture Oral Dosage and Nutraceutical forms by acting as a filler-binder while serving as a fibre source for your customers.
Pharmacopoeia Ref : EP/USP/JP
Technical Specs : Not Available
Ingredient(s) : Microcrystalline Cellulose Excipients
Dosage Form : Syrup
Grade : Not Available
Application : Taste Masking
Excipient Details : Act as a bulk sweetener and vehicle in liquid dosage forms and as a humectant in semi solids.
Dosage Form : Tablet
Grade : Not Available
Dosage Form : Injectable / Parenteral
Grade : Not Available
Application : Parenteral
Excipient Details : A pyrogen-free sorbitol used as a carbohydrate source and osmotic diuretic agent in large volume parenteral injectables.
Dosage Form : Tablet
Grade : Not Available
Application : Disintegrants & Superdisintegrants
Excipient Details : It is a superdisintegrant that provides an efficient disintegration at low level of use
Dosage Form : Tablet
Grade : Not Available
Application : Fillers, Diluents & Binders
Excipient Details : Used as disintegrants, fillers and binders (once cooked) in nutraceutical and pharmaceutical dosage forms.
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
69
PharmaCompass offers a list of Propranolol Hydrochloride API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Propranolol Hydrochloride manufacturer or Propranolol Hydrochloride supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Propranolol Hydrochloride manufacturer or Propranolol Hydrochloride supplier.
PharmaCompass also assists you with knowing the Propranolol Hydrochloride API Price utilized in the formulation of products. Propranolol Hydrochloride API Price is not always fixed or binding as the Propranolol Hydrochloride Price is obtained through a variety of data sources. The Propranolol Hydrochloride Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Inderal manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Inderal, including repackagers and relabelers. The FDA regulates Inderal manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Inderal API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Inderal manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Inderal supplier is an individual or a company that provides Inderal active pharmaceutical ingredient (API) or Inderal finished formulations upon request. The Inderal suppliers may include Inderal API manufacturers, exporters, distributors and traders.
click here to find a list of Inderal suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Inderal DMF (Drug Master File) is a document detailing the whole manufacturing process of Inderal active pharmaceutical ingredient (API) in detail. Different forms of Inderal DMFs exist exist since differing nations have different regulations, such as Inderal USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Inderal DMF submitted to regulatory agencies in the US is known as a USDMF. Inderal USDMF includes data on Inderal's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Inderal USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Inderal suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Inderal Drug Master File in Japan (Inderal JDMF) empowers Inderal API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Inderal JDMF during the approval evaluation for pharmaceutical products. At the time of Inderal JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Inderal suppliers with JDMF on PharmaCompass.
A Inderal CEP of the European Pharmacopoeia monograph is often referred to as a Inderal Certificate of Suitability (COS). The purpose of a Inderal CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Inderal EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Inderal to their clients by showing that a Inderal CEP has been issued for it. The manufacturer submits a Inderal CEP (COS) as part of the market authorization procedure, and it takes on the role of a Inderal CEP holder for the record. Additionally, the data presented in the Inderal CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Inderal DMF.
A Inderal CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Inderal CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Inderal suppliers with CEP (COS) on PharmaCompass.
A Inderal written confirmation (Inderal WC) is an official document issued by a regulatory agency to a Inderal manufacturer, verifying that the manufacturing facility of a Inderal active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Inderal APIs or Inderal finished pharmaceutical products to another nation, regulatory agencies frequently require a Inderal WC (written confirmation) as part of the regulatory process.
click here to find a list of Inderal suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Inderal as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Inderal API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Inderal as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Inderal and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Inderal NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Inderal suppliers with NDC on PharmaCompass.
Inderal Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Inderal GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Inderal GMP manufacturer or Inderal GMP API supplier for your needs.
A Inderal CoA (Certificate of Analysis) is a formal document that attests to Inderal's compliance with Inderal specifications and serves as a tool for batch-level quality control.
Inderal CoA mostly includes findings from lab analyses of a specific batch. For each Inderal CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Inderal may be tested according to a variety of international standards, such as European Pharmacopoeia (Inderal EP), Inderal JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Inderal USP).

