
Synopsis
Synopsis
0
VMF
0
US Patents
0
US Exclusivities
0
Health Canada Patents
Annual Reports
NA
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. 5-ismn
2. 5-ismn Durules
3. Elantan
4. Imdur
5. Ismo
6. Isosorbide-5-mononitrate
7. Isosorbide-5-nitrate
8. Monizid
9. Mono Mac 50d
10. Monocinque
11. Monoket
12. Mononit
13. Olicard 40
14. Olicard-retard
1. Isosorbide 5-mononitrate
2. 16051-77-7
3. Imdur
4. Corangin
5. Monosorbitrate
6. Monoket
7. Elantan
8. Mononit
9. Isosorbide-5-mononitrate
10. Isosorbide 5-nitrate
11. Ismn
12. Ismo
13. Monolong
14. Monopront
15. Monosordil
16. Nitramin
17. Orasorbil
18. Promocard
19. Sigacora
20. Sorbimon
21. Turimonit
22. Vasdilat
23. Duride
24. Imtrate
25. Ismexin
26. Medocor
27. Monicor
28. Olicard
29. Pertil
30. Uniket
31. Ismox
32. Monodur Durules
33. Mono Corax
34. Monisid
35. Monocord
36. Isosorbidi Mononitras [latin]
37. Isosorbidi Mononitras
38. Mononitrate D'isosorbide [french]
39. Is 5-mn
40. Mononitrate D'isosorbide
41. Mononitrato De Isosorbida [spanish]
42. 1,4:3,6-dianhydro-d-glucitol 5-nitrate
43. Mononitrato De Isosorbida
44. Iso-5-mononitrate
45. Ahr-4698
46. Is-5-mn
47. D-glucitol, 1,4:3,6-dianhydro-, 5-nitrate
48. Is-5mn
49. 1,4:3,6-dianhydro-5-o-nitro-d-erythro-hexitol
50. Monizid
51. Bm 22.145
52. Bm-22-145
53. Vasotrate
54. Epicordin
55. Monoclair
56. Monotrate
57. Olicardin
58. Percorina
59. Edistol
60. Etimonis
61. Isomonat
62. Isomonit
63. Multitab
64. Titarane
65. Conpin
66. Imazin
67. Imodur
68. Isomon
69. Iturol
70. Monis
71. Nitex
72. Plodin
73. Ihd
74. Monosigma
75. Elantan Retard
76. Mono-sanorania
77. Monoket Retard
78. Nsc-758619
79. Elantan Long
80. Imdur Durules
81. Corangin Sr
82. Mono Mack
83. Mono-mack
84. Conpin Retardkaps
85. Diluted Isosorbide Mononitrate
86. Fem-mono
87. Ismn Lannacher
88. Monoket Od
89. Ismn Apogepha
90. Ismn Heumann
91. Ismn Basics
92. Pentacard 20
93. [(3s,3ar,6r,6as)-3-hydroxy-2,3,3a,5,6,6a-hexahydrofuro[3,2-b]furan-6-yl] Nitrate
94. 1,4:3,6-dianhydro-5-o-nitro-d-glucitol
95. Mono Corax Retard
96. Chebi:6062
97. Ismn Hexal
98. Ismn Stada
99. Monocord 20
100. Monocord 40
101. Monolong 40
102. Monolong 60
103. Ismn Atid
104. Mononit 20
105. Mononit 40
106. Isopen-20
107. Monocord 50 Sr
108. Mononit Retard 50
109. 5-ismn
110. (3r,3as,6s,6ar)-6-hydroxy-hexahydrofuro[3,2-b]furan-3-yl Nitrate
111. Imdur 60
112. Ismn Abz
113. Lx1oh63030
114. Monit 20
115. Ismn Al
116. Ismo-20
117. Isosorbide-5-nitrate
118. Monocedocard
119. Bm-22145
120. Is 5mn
121. Monosorb
122. Bm-22.145
123. (3r,3as,6s,6ar)-6-hydroxyhexahydrofuro[3,2-b]furan-3-yl Nitrate
124. Bm 22.145is 5-mnahr-4698
125. Glucitol, 1,4:3,6-dianhydro-, 5-nitrate, D-
126. Monomax
127. Ahr 4698
128. Monosorb Xl 60
129. Ccris 1911
130. Imdur (tn)
131. Isosorbide Mononitrate (isosorbide 5-nitrate)
132. Ismo (tn)
133. Bm 22-145
134. Sr-05000001872
135. Einecs 240-197-2
136. Un3251
137. Brn 5851319
138. Leicester
139. Unii-lx1oh63030
140. Ncgc00159334-02
141. Isorbide Mononitrate
142. Mfcd00143462
143. Isosorbide-mononitrate
144. Isosobide-5-mononitrate
145. 5-isosorbide Mononitrate
146. Dsstox_cid_3176
147. Isosorbide Mononitrate,(s)
148. Chembl1311
149. Dsstox_rid_76905
150. Dsstox_gsid_23176
151. Isosorbide Mononitrate [usan:usp:inn:ban:jan]
152. Schembl26781
153. Gtpl7052
154. Dtxsid9023176
155. Niosh/lz4385560
156. Hms2093k07
157. Hms3715i08
158. Pharmakon1600-01503807
159. Hy-b0642
160. Zinc1849548
161. Isosorbide Mononitrate [inn]
162. Isosorbide Mononitrate [jan]
163. Tox21_111581
164. Isosorbide Mononitrate [usan]
165. Nsc758619
166. S4633
167. Isosorbide Mononitrate [vandf]
168. Akos005110986
169. Isosorbide Mononitrate [mart.]
170. Ac-8830
171. Ccg-213200
172. Cs-3495
173. Db01020
174. Isosorbide Mononitrate [who-dd]
175. Nsc 758619
176. Isosorbide Mononitrate (jp17/usp/inn)
177. Ncgc00167981-01
178. Ncgc00167981-03
179. D-1,4:3,6-dianhydroglucitol 5-nitrate
180. 1,4:3,6-dianhydrosorbitol 5-mononitrate
181. Sbi-0206724.p001
182. Cas-16051-77-7
183. Isosorbide Mononitrate [orange Book]
184. Bb 0261072
185. I0403
186. Isosorbide Dinitrate Isosorbide-5-mononitrate
187. Isosorbide Mononitrate [usp Impurity]
188. Lz43855600
189. C07714
190. D00630
191. Diluted Isosorbide Mononitrate [usp-rs]
192. W15583
193. Ab01563284_01
194. Q423401
195. Sr-05000001872-1
196. Sr-05000001872-2
197. W-107977
198. Brd-k82225283-001-01-5
199. Diluted Isosorbide Mononitrate [usp Monograph]
200. Isosobide-5-mononitrate [un3251] [flammable Solid]
201. Isosorbide Mononitrate 1.0 Mg/ml In Dimethyl Sulfoxide
202. Isosorbide Dinitrate Isosorbide-5-mononitrate [mi]
203. Isosorbide Dinitrate, Diluted Impurity C [ep Impurity]
204. [(3r,3as,6s,6ar)-6-hydroxy-2,3,3a,5,6,6a-hexahydrofuro[2,3-d]furan-3-yl] Nitrate
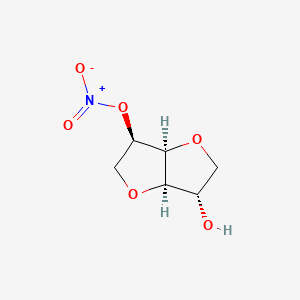
| Molecular Weight | 191.14 g/mol |
|---|---|
| Molecular Formula | C6H9NO6 |
| XLogP3 | -0.4 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 1 |
| Exact Mass | 191.04298701 g/mol |
| Monoisotopic Mass | 191.04298701 g/mol |
| Topological Polar Surface Area | 93.7 Ų |
| Heavy Atom Count | 13 |
| Formal Charge | 0 |
| Complexity | 216 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Isosorbide mononitrate |
| PubMed Health | Isosorbide Mononitrate (By mouth) |
| Drug Classes | Antianginal, Coronary Vasodilator |
| Drug Label | Isosorbide mononitrate (ISMN), an organic nitrate and the major biologically active metabolite of isosorbide dinitrate (ISDN), is a vasodilator with effects on both arteries and veins.Each tablet, for oral administration, contains either 30 mg, 60 mg... |
| Active Ingredient | Isosorbide mononitrate |
| Dosage Form | Tablet, extended release; Tablet |
| Route | Oral |
| Strength | 30mg; 120mg; 60mg; 10mg; 20mg |
| Market Status | Prescription |
| Company | Vintage Pharms; Ani Pharms; Torrent Pharms; Hikma Pharms; Dexcel; Nesher Pharms; Kremers Urban Pharms; Actavis Elizabeth |
| 2 of 4 | |
|---|---|
| Drug Name | Monoket |
| PubMed Health | Isosorbide Mononitrate (By mouth) |
| Drug Classes | Antianginal, Coronary Vasodilator |
| Drug Label | monoket, an organic nitrate, is a vasodilator with effects on both arteries and veins. The empirical formula is C6H9NO6 and the molecular weight is 191.14. The chemical name for monoket is 1,4:3,6-Dianhydro-D-glucitol 5-nitrate and the compound h... |
| Active Ingredient | Isosorbide mononitrate |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 10mg; 20mg |
| Market Status | Prescription |
| Company | Kremers Urban Pharms |
| 3 of 4 | |
|---|---|
| Drug Name | Isosorbide mononitrate |
| PubMed Health | Isosorbide Mononitrate (By mouth) |
| Drug Classes | Antianginal, Coronary Vasodilator |
| Drug Label | Isosorbide mononitrate (ISMN), an organic nitrate and the major biologically active metabolite of isosorbide dinitrate (ISDN), is a vasodilator with effects on both arteries and veins.Each tablet, for oral administration, contains either 30 mg, 60 mg... |
| Active Ingredient | Isosorbide mononitrate |
| Dosage Form | Tablet, extended release; Tablet |
| Route | Oral |
| Strength | 30mg; 120mg; 60mg; 10mg; 20mg |
| Market Status | Prescription |
| Company | Vintage Pharms; Ani Pharms; Torrent Pharms; Hikma Pharms; Dexcel; Nesher Pharms; Kremers Urban Pharms; Actavis Elizabeth |
| 4 of 4 | |
|---|---|
| Drug Name | Monoket |
| PubMed Health | Isosorbide Mononitrate (By mouth) |
| Drug Classes | Antianginal, Coronary Vasodilator |
| Drug Label | monoket, an organic nitrate, is a vasodilator with effects on both arteries and veins. The empirical formula is C6H9NO6 and the molecular weight is 191.14. The chemical name for monoket is 1,4:3,6-Dianhydro-D-glucitol 5-nitrate and the compound h... |
| Active Ingredient | Isosorbide mononitrate |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 10mg; 20mg |
| Market Status | Prescription |
| Company | Kremers Urban Pharms |
Isosorbide mononitrate is indicated for the prevention and management of angina pectoris due to coronary artery disease. The onset of action of oral isosorbide mononitrate is not sufficiently rapid to be useful in aborting an acute anginal episode.
FDA Label
Isosorbide mononitrate is an anti-anginal agent and vasodilator that relaxes vascular smooth muscle to prevent and manage angina pectoris. The pharmacological action is mediated by the active metabolite, [nitric oxide], which is released when isosorbide mononitrate is metabolized. Nitric oxide works on both arteries and veins, but predominantly veins: by relaxing veins and reducing the central venous pressure, nitric oxide causes venous pooling and a decrease in the venous return to the heart, thus decreasing cardiac preload. In healthy subjects, the stroke volume is decreased and venous pooling can occur in the standing posture, leading to postural hypotension and dizziness. At therapeutic doses of isosorbide mononitrate, nitric oxide has a bigger effect on larger muscular arteries over small resistance arteries. Arterial relaxation leads to reduced systemic vascular resistance and systolic blood (aortic) pressure, decreasing to decreased cardiac afterload. The direct dilator effect on coronary arteries opposes the coronary artery spasm in variant angina or angina pectoris. At larger doses, nitric oxide causes the resistance arteries and arterioles to dilate, reducing arterial pressure via coronary vasodilatation. This leads to increased coronary blood flow. Reduced cardiac preload and afterload caused by nitric oxide causes a reduction in myocardial oxygen consumption; decreased myocardial oxygen demand, along with increased coronary blood flow, leads to an increased in the oxygen content of coronary sinus blood and the relief from ischemia. The end effect of isosorbide mononitrate include decreased cardiac oxygen consumption, redistribution coronary flow toward ischemic areas via collaterals, and the relief of coronary spasms. Nitric oxide can also increase the rate of relaxation of cardiac muscles, which is an effect outside of vascular smooth muscles. Organic nitrates can also relax other types of smooth muscles, including esophageal and biliary smooth muscle. The anti-anginal activity of isosorbide mononitrate was observed about 1 hour after dosing, and the peak effect was achieved from 1-4 hours after dosing. The duration of anti-anginal action of at least 12 hours was observed with an asymmetrical dosing regimen.
Nitric Oxide Donors
A diverse group of agents, with unique chemical structures and biochemical requirements, which generate NITRIC OXIDE. These compounds have been used in the treatment of cardiovascular diseases and the management of acute myocardial infarction, acute and chronic congestive heart failure, and surgical control of blood pressure. (Adv Pharmacol 1995;34:361-81) (See all compounds classified as Nitric Oxide Donors.)
Vasodilator Agents
Drugs used to cause dilation of the blood vessels. (See all compounds classified as Vasodilator Agents.)
C - Cardiovascular system
C01 - Cardiac therapy
C01D - Vasodilators used in cardiac diseases
C01DA - Organic nitrates
C01DA14 - Isosorbide mononitrate
Absorption
Upon oral administration, isosorbide mononitrate is rapidly and completely absorbed from the gastrointestinal tract. Isosorbide mononitrate has a dose-linear kinetics and the absolute bioavailability is nearly 100%. The Cmax is reached within 30 to 60 minutes following administration.
Route of Elimination
In a human radio-labelled drug study, about 93% of the total dose was excreted in the urine within 48 hours. Following oral administration of 20 mg, only 2% of isosorbide mononitrate was excreted unchanged in the urine within 24 hours. Among the excreted dose, nearly half of the dose was found de-nitrated in urine as isosorbide and sorbitol: approximately 30% is excreted as isosorbide and about 17% is the 2-glucuronide of mononitrate. These metabolites were not vasoactive or pharmacologically active. Renal excretion was complete after 5 days, and fecal excretion accounted for only 1% of drug elimination.
Volume of Distribution
The volume of distribution is approximately 0.6 L/kg, which is approximately the volume of total body water.
Clearance
The total body clearance is 115-120 mL/min.
Isosorbide mononitrate is not subject to first pass metabolism in human liver. Detectable metabolites include isosorbide, sorbitol, and 2-glucuronide of mononitrate, which are pharmacologically inactive.
The elimination half-life of isosorbide mononitrate is about 5 hours. The elimination half-life of its metabolites, isosorbide and 2-glucuronide of mononitrate, are 8 hours and 6 hours, respectively.
Isosorbide mononitrate acts as a prodrug for nitric oxide (NO), which is a potent vasodilator gas that is released when the drug is metabolized. NO activates soluble guanylyl cyclase in vascular endothelial cells, which increases the intracellular concentrations of cyclic GMP (cGMP). cGMP activates cGMP-dependent protein kinases, such as protein kinase G and I, which activates the downstream intracellular cascades. The downstream cascade results in reduced intracellular concentrations of calcium, caused by processes including inhibition of IP3-mediated pathway, phosphorylation of big calcium-activated potassium channel leading to cell hyperpolarization and reduced calcium influx, and increased calcium efflux via the Ca2+-ATPase-pump. Reduced intracellular calcium concentrations lead to the dephosphorylation of myosin light chains and the relaxation of smooth muscle cells.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
61
PharmaCompass offers a list of Isosorbide Mononitrate API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Isosorbide Mononitrate manufacturer or Isosorbide Mononitrate supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Isosorbide Mononitrate manufacturer or Isosorbide Mononitrate supplier.
PharmaCompass also assists you with knowing the Isosorbide Mononitrate API Price utilized in the formulation of products. Isosorbide Mononitrate API Price is not always fixed or binding as the Isosorbide Mononitrate Price is obtained through a variety of data sources. The Isosorbide Mononitrate Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Imdur manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Imdur, including repackagers and relabelers. The FDA regulates Imdur manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Imdur API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Imdur manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Imdur supplier is an individual or a company that provides Imdur active pharmaceutical ingredient (API) or Imdur finished formulations upon request. The Imdur suppliers may include Imdur API manufacturers, exporters, distributors and traders.
click here to find a list of Imdur suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Imdur DMF (Drug Master File) is a document detailing the whole manufacturing process of Imdur active pharmaceutical ingredient (API) in detail. Different forms of Imdur DMFs exist exist since differing nations have different regulations, such as Imdur USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Imdur DMF submitted to regulatory agencies in the US is known as a USDMF. Imdur USDMF includes data on Imdur's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Imdur USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Imdur suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Imdur Drug Master File in Japan (Imdur JDMF) empowers Imdur API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Imdur JDMF during the approval evaluation for pharmaceutical products. At the time of Imdur JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Imdur suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Imdur Drug Master File in Korea (Imdur KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Imdur. The MFDS reviews the Imdur KDMF as part of the drug registration process and uses the information provided in the Imdur KDMF to evaluate the safety and efficacy of the drug.
After submitting a Imdur KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Imdur API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Imdur suppliers with KDMF on PharmaCompass.
A Imdur CEP of the European Pharmacopoeia monograph is often referred to as a Imdur Certificate of Suitability (COS). The purpose of a Imdur CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Imdur EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Imdur to their clients by showing that a Imdur CEP has been issued for it. The manufacturer submits a Imdur CEP (COS) as part of the market authorization procedure, and it takes on the role of a Imdur CEP holder for the record. Additionally, the data presented in the Imdur CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Imdur DMF.
A Imdur CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Imdur CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Imdur suppliers with CEP (COS) on PharmaCompass.
A Imdur written confirmation (Imdur WC) is an official document issued by a regulatory agency to a Imdur manufacturer, verifying that the manufacturing facility of a Imdur active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Imdur APIs or Imdur finished pharmaceutical products to another nation, regulatory agencies frequently require a Imdur WC (written confirmation) as part of the regulatory process.
click here to find a list of Imdur suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Imdur as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Imdur API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Imdur as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Imdur and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Imdur NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Imdur suppliers with NDC on PharmaCompass.
Imdur Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Imdur GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Imdur GMP manufacturer or Imdur GMP API supplier for your needs.
A Imdur CoA (Certificate of Analysis) is a formal document that attests to Imdur's compliance with Imdur specifications and serves as a tool for batch-level quality control.
Imdur CoA mostly includes findings from lab analyses of a specific batch. For each Imdur CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Imdur may be tested according to a variety of international standards, such as European Pharmacopoeia (Imdur EP), Imdur JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Imdur USP).

