



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
Other Suppliers
0

USA (Orange Book)

Europe

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
U.S. Medicaid
Annual Reports
0
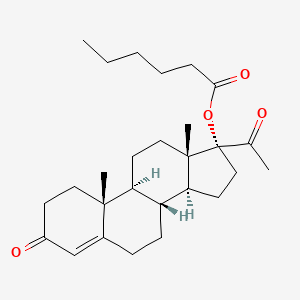

1. 17 Alpha Hydroxy Progesterone Caproate
2. 17 Alpha Hydroxyprogesterone Caproate
3. 17 Alpha Oxyprogesterone Capronate
4. 17 Alpha-hydroxyprogesterone Caproate
5. 17 Alpha-oxyprogesterone Capronate
6. 17 Hydroxyprogesterone Capronate
7. 17-alpha-hydroxy-progesterone Caproate
8. 17-hydroxyprogesterone Capronate
9. Caproate, 17-alpha-hydroxy-progesterone
10. Delalutin
11. Hydroxyprogesterone Hexanoate
12. Makena
13. Neolutin
14. Oxyprogesterone Caproate
15. Prolutin Depot
16. Proluton Depot
1. 630-56-8
2. Delalutin
3. Primolut Depot
4. Hydroxyprogesterone Hexanoate
5. Hormofort
6. Proge
7. Syngynon
8. Depo-proluton
9. Proluton Depot
10. Hylutin
11. Teralutil
12. Makena
13. Estralutin
14. Hyproval
15. Kaprogest
16. Neolutin
17. Lutate
18. Relutin
19. Progesterone Caproate
20. Neolutin Forte
21. 17-caproxyprogesterone
22. Idrogestene
23. Duraluton
24. Hyroxon
25. Luteocrin
26. Lutopron
27. Luteocrin Depot
28. Hyproval-pa
29. 17alpha-hydroxyprogesterone Caproate
30. Nsc-17592
31. 17-alpha-hydroxy-progesterone Caproate
32. Corlutin L.a.
33. Gesterol La 250
34. 17-ohpc
35. Procyte Depo
36. Progesterone Retard Pharlon
37. 17alpha-caproyloxypregn-4-ene-3,20-dione
38. 17-alpha-hydroxyprogesterone Caproate
39. 17-((1-oxohexyl)oxy)pregn-4-ene-3,20-dione
40. Mls000028438
41. 17a-hydroxyprogesterone Caproate
42. Oxiprogesterone Caproate
43. 17 Alpha-hydroxyprogesterone Caproate
44. 17alpha-hydroxyprogesterone Hexanoate
45. 17.alpha.-caproyloxy-p4
46. Progesterone, 17-hydroxy-, Hexanoate
47. Smr000058336
48. Mls001148643
49. Chebi:5812
50. 17-hydroxypregn-4-ene-3,20-dione Hexanoate
51. 17a-hydroxyprogesterone Hexanoate
52. Pregn-4-ene-3,20-dione, 17-hydroxy-, Hexanoate
53. Pregn-4-ene-3,20-dione, 17-((1-oxohexyl)oxy)-
54. 17.alpha.-hydroxyprogesterone Caproate
55. 276f2o42f5
56. (8r,9s,10r,13s,14s,17r)-17-acetyl-10,13-dimethyl-3-oxo-2,3,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1h-cyclopenta[a]phenanthren-17-yl Hexanoate
57. 17.alpha.-hydroxyprogesterone Hexanoate
58. Ncgc00021268-03
59. 17.alpha.-hydroxyprogesterone N-caproate
60. 3,20-dioxopregn-4-en-17.alpha.-yl Caproate
61. Dsstox_cid_23915
62. Dsstox_rid_80089
63. Dsstox_gsid_43915
64. [(8r,9s,10r,13s,14s,17r)-17-acetyl-10,13-dimethyl-3-oxo-2,6,7,8,9,11,12,14,15,16-decahydro-1h-cyclopenta[a]phenanthren-17-yl] Hexanoate
65. 17.alpha.-hydroxypregn-4-ene-3,20-dione Hexanoate
66. Pregn-4-ene-3,20-dione, 17-[(1-oxohexyl)oxy]-
67. Gestiva
68. Hexanoic Acid, Ester With 17-hydroxypregn-4-ene-3,20-dione
69. Delalutin (tn)
70. Cas-630-56-8
71. 17
72. A-hydroxyprogesterone Caproate
73. Idroprogesterone Caproato
74. Hydroxyprogesteroni Caproas
75. Lewntogest
76. Caproate D'hydroxyprogesterone
77. Caproato De Hidroxiprogesterona
78. Unii-276f2o42f5
79. Idroprogesterone Caproato [dcit]
80. 17
81. A-hydroxyprogesterone Hexanoate
82. Hyproval P.a.
83. Einecs 211-138-8
84. Hylutin (tn)
85. Makena (tn)
86. 17-alpha-hydroxyprogesterone Hexanoate
87. 17alpha-hydroxyprogesterone N-caproate
88. Hydroxyprogesteroni Caproas [inn-latin]
89. Progesterone, Hexanoate
90. 17-alpha-hydroxy Progesterone N-caproate
91. 17-caproyloxyprogesterone
92. 3,20-dioxo-4-pregnen-17alpha-yl Hexanoat
93. 3,20-dioxopregn-4-en-17alpha-yl Caproate
94. Caproate D'hydroxyprogesterone [inn-french]
95. Hydroxyprogesterone Caproate [usp:inn:jan]
96. Opera_id_1479
97. 17alpha-hydroxyprogesterone-17alpha Caproate
98. 17-alpha-hexanoyloxypregn-4-ene-3,20-dione
99. Caproato De Hidroxiprogesterona [inn-spanish]
100. Deluteval 2x (salt/mix)
101. Schembl5330
102. Mls002207289
103. 17alpha-caproyloxyprogesterone
104. 17-hydroxyprogesterone-caproate
105. Caproic Acid Hydroxyprogesterone
106. Cid_169870
107. Chembl1200848
108. Dtxsid6043915
109. Bdbm70293
110. 17alpha-hydroxyprogesteron-caproat
111. Hms2230l08
112. Bcp16081
113. Hy-b0742
114. Nsc17592
115. Zinc4083606
116. Tox21_113502
117. Tox21_302333
118. 17-alpha Hydroxyprogesterone Caproate
119. Mfcd00072134
120. S4674
121. Akos005267159
122. Tox21_113502_1
123. 17 Alpha -hydroxyprogesterone Caproate
124. Ccg-268987
125. Db06789
126. Gs-3233
127. Ncgc00021268-04
128. Ncgc00255784-01
129. (1s,11s,15s,2r,10r,14r)-14-acetyl-2,15-dimethyl-5-oxotetracyclo[8.7.0.0<2,7>.0 <11,15>]heptadec-6-en-14-yl Hexanoate
130. Hydroxyprogesterone Caproate [inn]
131. Hydroxyprogesterone Caproate [jan]
132. Pregn-4-ene-3, 17-hydroxy-, Hexanoate
133. 17alpha-hexanoyloxy-4pregnene-3,20-dione
134. Hydroxyprogesterone Caproate (jan/usp/inn)
135. Hydroxyprogesterone Caproate [mart.]
136. Hydroxyprogesterone Caproate [vandf]
137. Pregn-4-ene-3, 17-[(1-oxohexyl)oxy]-
138. 17-hydroxypregn-4-ene-3,20-dione Caproate
139. H0994
140. Hydroxyprogesterone Caproate [usp-rs]
141. Hydroxyprogesterone Caproate [who-dd]
142. 17alpha-hydroxyprogesterone Hexanoate, >=98%
143. C08148
144. D00949
145. H10189
146. 17-.alpha.-hexanoyloxypregn-4-ene-3,20-dione
147. 17alpha-hydroxypregn-4-ene-3,20-diene Caproate
148. 17alpha-hydroxyprogesterone Caproate [progestins]
149. 3,20-dioxopregn-4-en-17.alpha.-yl Hexanoate #
150. 630h568
151. A834188
152. Hydroxyprogesterone Caproate [orange Book]
153. Sr-01000003075
154. 17.alpha.-hydroxypregn-4-ene-3,20-dione Caproate
155. 17.alpha.-hydroxyprogesterone Caproate [mi]
156. Hydroxyprogesterone Caproate [usp Monograph]
157. Q-201222
158. Q3792032
159. Sr-01000003075-3
160. Hydroxyprogesterone Caproate, United States Pharmacopeia (usp) Reference Standard
161. (1s,2r,10r,11s,14r,15s)-14-acetyl-2,15-dimethyl-5-oxotetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadec-6-en-14-yl Hexanoate
162. [(10r,13s,17r)-17-acetyl-10,13-dimethyl-3-oxo-2,6,7,8,9,11,12,14,15,16-decahydro-1h-cyclopenta[a]phenanthren-17-yl] Hexanoate
163. [(8r,9s,10r,13s,14s,17r)-17-acetyl-10,13-dimethyl-3-oxo-2,6,7,8,9,11,12,14,15,16-decahydro-1h-cyclopenta[a]phenanthren-17-yl] Hexanoate;17-caproxyprogesterone
| Molecular Weight | 428.6 g/mol |
|---|---|
| Molecular Formula | C27H40O4 |
| XLogP3 | 5.7 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 7 |
| Exact Mass | 428.29265975 g/mol |
| Monoisotopic Mass | 428.29265975 g/mol |
| Topological Polar Surface Area | 60.4 Ų |
| Heavy Atom Count | 31 |
| Formal Charge | 0 |
| Complexity | 797 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 6 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Makena |
| PubMed Health | Hydroxyprogesterone |
| Drug Classes | Endocrine-Metabolic Agent, Hydroxyprogesterone |
| Drug Label | The active pharmaceutical ingredient in Makena is hydroxyprogesterone caproate.The chemical name for hydroxyprogesterone caproate is pregn-4-ene-3,20-dione, 17[(1-oxohexyl)oxy]. It has an empirical formula of C27H40O4 and a molecular weight of 428.60... |
| Active Ingredient | Hydroxyprogesterone caproate |
| Dosage Form | Solution |
| Route | Intramuscular |
| Strength | 1250mg/5ml (250mg/ml) |
| Market Status | Prescription |
| Company | Lumara Health |
| 2 of 2 | |
|---|---|
| Drug Name | Makena |
| PubMed Health | Hydroxyprogesterone |
| Drug Classes | Endocrine-Metabolic Agent, Hydroxyprogesterone |
| Drug Label | The active pharmaceutical ingredient in Makena is hydroxyprogesterone caproate.The chemical name for hydroxyprogesterone caproate is pregn-4-ene-3,20-dione, 17[(1-oxohexyl)oxy]. It has an empirical formula of C27H40O4 and a molecular weight of 428.60... |
| Active Ingredient | Hydroxyprogesterone caproate |
| Dosage Form | Solution |
| Route | Intramuscular |
| Strength | 1250mg/5ml (250mg/ml) |
| Market Status | Prescription |
| Company | Lumara Health |
Hydroxyprogesterone caproate is indicated for the prevention of spontaneous preterm births in singleton pregnancies in women who have previously had a spontaneous preterm birth. (1)
FDA Label
No specific pharmacodynamic studies have been performed to assess hydroxyprogesterone caproate injections. (4) However, the mechanism of action is likely related to increased interaction between progesterone and progesterone receptors. (5)
Progestins
Compounds that interact with PROGESTERONE RECEPTORS in target tissues to bring about the effects similar to those of PROGESTERONE. Primary actions of progestins, including natural and synthetic steroids, are on the UTERUS and the MAMMARY GLAND in preparation for and in maintenance of PREGNANCY. (See all compounds classified as Progestins.)
Estrogen Antagonists
Compounds which inhibit or antagonize the action or biosynthesis of estrogenic compounds. (See all compounds classified as Estrogen Antagonists.)
Absorption
Absorption of 17-hydroxyprogesteron caproate is slow, occurring over a long period of time. (3)
Route of Elimination
Following intramuscular injection, approximately 50% of hydroxyprogesterone caproate metabolites are eliminated in the feces, while approximately 30% of metabolites are eliminated in the urine. (3)
Volume of Distribution
Hydroxyprogesterone caproate has a high volume of distribution. (3)
Clearance
Clearance is highly variable from patient to patient. (3)
The main enzymes involved in metabolism of hydroxyprogesterone caproate are cytochrome P450 (CYP) 3A4 and to a lesser extent CYP3A5. (3)
Half-life = 16 days (6 days). (3)
The mechanism by which progesterone prevents preterm birth is not well understood, but many pathways are likely involved. (1) Progesterone plays a vital role in regulation of the female reproductive system and is important for successful implantation of the embryo and maintenance of pregnancy. It acts by binding to progesterone receptors in the uterus, ovaries, breasts and in the central nervous system. These receptors exist in 2 isoforms, PR-A and PR-B. Progesterone binding to these receptors ultimately leads to regulation of gene transcription. (2) This results in an anti-inflammatory effect which blunts the proinflammatory state that occurs with initiation of labor, and maintains uterine queiscence by stabilizing progesterone acting on the myometrium. (2)


