
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
KDMF
0
VMF
0
Canada
0
Australia
0
South Africa
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
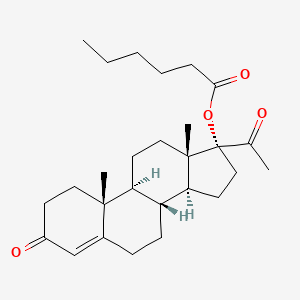

1. 17 Alpha Hydroxy Progesterone Caproate
2. 17 Alpha Hydroxyprogesterone Caproate
3. 17 Alpha Oxyprogesterone Capronate
4. 17 Alpha-hydroxyprogesterone Caproate
5. 17 Alpha-oxyprogesterone Capronate
6. 17 Hydroxyprogesterone Capronate
7. 17-alpha-hydroxy-progesterone Caproate
8. 17-hydroxyprogesterone Capronate
9. Caproate, 17-alpha-hydroxy-progesterone
10. Delalutin
11. Hydroxyprogesterone Hexanoate
12. Makena
13. Neolutin
14. Oxyprogesterone Caproate
15. Prolutin Depot
16. Proluton Depot
1. 630-56-8
2. Delalutin
3. Primolut Depot
4. Hydroxyprogesterone Hexanoate
5. Hormofort
6. Proge
7. Syngynon
8. Depo-proluton
9. Proluton Depot
10. Hylutin
11. Teralutil
12. Makena
13. Estralutin
14. Hyproval
15. Kaprogest
16. Neolutin
17. Lutate
18. Relutin
19. Progesterone Caproate
20. Neolutin Forte
21. 17-caproxyprogesterone
22. Idrogestene
23. Duraluton
24. Hyroxon
25. Luteocrin
26. Lutopron
27. Luteocrin Depot
28. Hyproval-pa
29. 17alpha-hydroxyprogesterone Caproate
30. Nsc-17592
31. 17-alpha-hydroxy-progesterone Caproate
32. Corlutin L.a.
33. Gesterol La 250
34. 17-ohpc
35. Procyte Depo
36. Progesterone Retard Pharlon
37. 17alpha-caproyloxypregn-4-ene-3,20-dione
38. 17-alpha-hydroxyprogesterone Caproate
39. 17-((1-oxohexyl)oxy)pregn-4-ene-3,20-dione
40. Mls000028438
41. 17a-hydroxyprogesterone Caproate
42. Oxiprogesterone Caproate
43. 17 Alpha-hydroxyprogesterone Caproate
44. 17alpha-hydroxyprogesterone Hexanoate
45. 17.alpha.-caproyloxy-p4
46. Progesterone, 17-hydroxy-, Hexanoate
47. Smr000058336
48. Mls001148643
49. Chebi:5812
50. 17-hydroxypregn-4-ene-3,20-dione Hexanoate
51. 17a-hydroxyprogesterone Hexanoate
52. Pregn-4-ene-3,20-dione, 17-hydroxy-, Hexanoate
53. Pregn-4-ene-3,20-dione, 17-((1-oxohexyl)oxy)-
54. 17.alpha.-hydroxyprogesterone Caproate
55. 276f2o42f5
56. (8r,9s,10r,13s,14s,17r)-17-acetyl-10,13-dimethyl-3-oxo-2,3,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1h-cyclopenta[a]phenanthren-17-yl Hexanoate
57. 17.alpha.-hydroxyprogesterone Hexanoate
58. Ncgc00021268-03
59. 17.alpha.-hydroxyprogesterone N-caproate
60. 3,20-dioxopregn-4-en-17.alpha.-yl Caproate
61. Dsstox_cid_23915
62. Dsstox_rid_80089
63. Dsstox_gsid_43915
64. [(8r,9s,10r,13s,14s,17r)-17-acetyl-10,13-dimethyl-3-oxo-2,6,7,8,9,11,12,14,15,16-decahydro-1h-cyclopenta[a]phenanthren-17-yl] Hexanoate
65. 17.alpha.-hydroxypregn-4-ene-3,20-dione Hexanoate
66. Pregn-4-ene-3,20-dione, 17-[(1-oxohexyl)oxy]-
67. Gestiva
68. Hexanoic Acid, Ester With 17-hydroxypregn-4-ene-3,20-dione
69. Delalutin (tn)
70. Cas-630-56-8
71. 17
72. A-hydroxyprogesterone Caproate
73. Idroprogesterone Caproato
74. Hydroxyprogesteroni Caproas
75. Lewntogest
76. Caproate D'hydroxyprogesterone
77. Caproato De Hidroxiprogesterona
78. Unii-276f2o42f5
79. Idroprogesterone Caproato [dcit]
80. 17
81. A-hydroxyprogesterone Hexanoate
82. Hyproval P.a.
83. Einecs 211-138-8
84. Hylutin (tn)
85. Makena (tn)
86. 17-alpha-hydroxyprogesterone Hexanoate
87. 17alpha-hydroxyprogesterone N-caproate
88. Hydroxyprogesteroni Caproas [inn-latin]
89. Progesterone, Hexanoate
90. 17-alpha-hydroxy Progesterone N-caproate
91. 17-caproyloxyprogesterone
92. 3,20-dioxo-4-pregnen-17alpha-yl Hexanoat
93. 3,20-dioxopregn-4-en-17alpha-yl Caproate
94. Caproate D'hydroxyprogesterone [inn-french]
95. Hydroxyprogesterone Caproate [usp:inn:jan]
96. Opera_id_1479
97. 17alpha-hydroxyprogesterone-17alpha Caproate
98. 17-alpha-hexanoyloxypregn-4-ene-3,20-dione
99. Caproato De Hidroxiprogesterona [inn-spanish]
100. Deluteval 2x (salt/mix)
101. Schembl5330
102. Mls002207289
103. 17alpha-caproyloxyprogesterone
104. 17-hydroxyprogesterone-caproate
105. Caproic Acid Hydroxyprogesterone
106. Cid_169870
107. Chembl1200848
108. Dtxsid6043915
109. Bdbm70293
110. 17alpha-hydroxyprogesteron-caproat
111. Hms2230l08
112. Bcp16081
113. Hy-b0742
114. Nsc17592
115. Zinc4083606
116. Tox21_113502
117. Tox21_302333
118. 17-alpha Hydroxyprogesterone Caproate
119. Mfcd00072134
120. S4674
121. Akos005267159
122. Tox21_113502_1
123. 17 Alpha -hydroxyprogesterone Caproate
124. Ccg-268987
125. Db06789
126. Gs-3233
127. Ncgc00021268-04
128. Ncgc00255784-01
129. (1s,11s,15s,2r,10r,14r)-14-acetyl-2,15-dimethyl-5-oxotetracyclo[8.7.0.0<2,7>.0 <11,15>]heptadec-6-en-14-yl Hexanoate
130. Hydroxyprogesterone Caproate [inn]
131. Hydroxyprogesterone Caproate [jan]
132. Pregn-4-ene-3, 17-hydroxy-, Hexanoate
133. 17alpha-hexanoyloxy-4pregnene-3,20-dione
134. Hydroxyprogesterone Caproate (jan/usp/inn)
135. Hydroxyprogesterone Caproate [mart.]
136. Hydroxyprogesterone Caproate [vandf]
137. Pregn-4-ene-3, 17-[(1-oxohexyl)oxy]-
138. 17-hydroxypregn-4-ene-3,20-dione Caproate
139. H0994
140. Hydroxyprogesterone Caproate [usp-rs]
141. Hydroxyprogesterone Caproate [who-dd]
142. 17alpha-hydroxyprogesterone Hexanoate, >=98%
143. C08148
144. D00949
145. H10189
146. 17-.alpha.-hexanoyloxypregn-4-ene-3,20-dione
147. 17alpha-hydroxypregn-4-ene-3,20-diene Caproate
148. 17alpha-hydroxyprogesterone Caproate [progestins]
149. 3,20-dioxopregn-4-en-17.alpha.-yl Hexanoate #
150. 630h568
151. A834188
152. Hydroxyprogesterone Caproate [orange Book]
153. Sr-01000003075
154. 17.alpha.-hydroxypregn-4-ene-3,20-dione Caproate
155. 17.alpha.-hydroxyprogesterone Caproate [mi]
156. Hydroxyprogesterone Caproate [usp Monograph]
157. Q-201222
158. Q3792032
159. Sr-01000003075-3
160. Hydroxyprogesterone Caproate, United States Pharmacopeia (usp) Reference Standard
161. (1s,2r,10r,11s,14r,15s)-14-acetyl-2,15-dimethyl-5-oxotetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadec-6-en-14-yl Hexanoate
162. [(10r,13s,17r)-17-acetyl-10,13-dimethyl-3-oxo-2,6,7,8,9,11,12,14,15,16-decahydro-1h-cyclopenta[a]phenanthren-17-yl] Hexanoate
163. [(8r,9s,10r,13s,14s,17r)-17-acetyl-10,13-dimethyl-3-oxo-2,6,7,8,9,11,12,14,15,16-decahydro-1h-cyclopenta[a]phenanthren-17-yl] Hexanoate;17-caproxyprogesterone
| Molecular Weight | 428.6 g/mol |
|---|---|
| Molecular Formula | C27H40O4 |
| XLogP3 | 5.7 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 7 |
| Exact Mass | 428.29265975 g/mol |
| Monoisotopic Mass | 428.29265975 g/mol |
| Topological Polar Surface Area | 60.4 Ų |
| Heavy Atom Count | 31 |
| Formal Charge | 0 |
| Complexity | 797 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 6 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Makena |
| PubMed Health | Hydroxyprogesterone |
| Drug Classes | Endocrine-Metabolic Agent, Hydroxyprogesterone |
| Drug Label | The active pharmaceutical ingredient in Makena is hydroxyprogesterone caproate.The chemical name for hydroxyprogesterone caproate is pregn-4-ene-3,20-dione, 17[(1-oxohexyl)oxy]. It has an empirical formula of C27H40O4 and a molecular weight of 428.60... |
| Active Ingredient | Hydroxyprogesterone caproate |
| Dosage Form | Solution |
| Route | Intramuscular |
| Strength | 1250mg/5ml (250mg/ml) |
| Market Status | Prescription |
| Company | Lumara Health |
| 2 of 2 | |
|---|---|
| Drug Name | Makena |
| PubMed Health | Hydroxyprogesterone |
| Drug Classes | Endocrine-Metabolic Agent, Hydroxyprogesterone |
| Drug Label | The active pharmaceutical ingredient in Makena is hydroxyprogesterone caproate.The chemical name for hydroxyprogesterone caproate is pregn-4-ene-3,20-dione, 17[(1-oxohexyl)oxy]. It has an empirical formula of C27H40O4 and a molecular weight of 428.60... |
| Active Ingredient | Hydroxyprogesterone caproate |
| Dosage Form | Solution |
| Route | Intramuscular |
| Strength | 1250mg/5ml (250mg/ml) |
| Market Status | Prescription |
| Company | Lumara Health |
Hydroxyprogesterone caproate is indicated for the prevention of spontaneous preterm births in singleton pregnancies in women who have previously had a spontaneous preterm birth. (1)
FDA Label
No specific pharmacodynamic studies have been performed to assess hydroxyprogesterone caproate injections. (4) However, the mechanism of action is likely related to increased interaction between progesterone and progesterone receptors. (5)
Progestins
Compounds that interact with PROGESTERONE RECEPTORS in target tissues to bring about the effects similar to those of PROGESTERONE. Primary actions of progestins, including natural and synthetic steroids, are on the UTERUS and the MAMMARY GLAND in preparation for and in maintenance of PREGNANCY. (See all compounds classified as Progestins.)
Estrogen Antagonists
Compounds which inhibit or antagonize the action or biosynthesis of estrogenic compounds. (See all compounds classified as Estrogen Antagonists.)
Absorption
Absorption of 17-hydroxyprogesteron caproate is slow, occurring over a long period of time. (3)
Route of Elimination
Following intramuscular injection, approximately 50% of hydroxyprogesterone caproate metabolites are eliminated in the feces, while approximately 30% of metabolites are eliminated in the urine. (3)
Volume of Distribution
Hydroxyprogesterone caproate has a high volume of distribution. (3)
Clearance
Clearance is highly variable from patient to patient. (3)
The main enzymes involved in metabolism of hydroxyprogesterone caproate are cytochrome P450 (CYP) 3A4 and to a lesser extent CYP3A5. (3)
Half-life = 16 days (6 days). (3)
The mechanism by which progesterone prevents preterm birth is not well understood, but many pathways are likely involved. (1) Progesterone plays a vital role in regulation of the female reproductive system and is important for successful implantation of the embryo and maintenance of pregnancy. It acts by binding to progesterone receptors in the uterus, ovaries, breasts and in the central nervous system. These receptors exist in 2 isoforms, PR-A and PR-B. Progesterone binding to these receptors ultimately leads to regulation of gene transcription. (2) This results in an anti-inflammatory effect which blunts the proinflammatory state that occurs with initiation of labor, and maintains uterine queiscence by stabilizing progesterone acting on the myometrium. (2)

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
53
PharmaCompass offers a list of Hydroxyprogesterone Caproate API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Hydroxyprogesterone Caproate manufacturer or Hydroxyprogesterone Caproate supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Hydroxyprogesterone Caproate manufacturer or Hydroxyprogesterone Caproate supplier.
PharmaCompass also assists you with knowing the Hydroxyprogesterone Caproate API Price utilized in the formulation of products. Hydroxyprogesterone Caproate API Price is not always fixed or binding as the Hydroxyprogesterone Caproate Price is obtained through a variety of data sources. The Hydroxyprogesterone Caproate Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Hydroxyprogesterone Caproate manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Hydroxyprogesterone Caproate, including repackagers and relabelers. The FDA regulates Hydroxyprogesterone Caproate manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Hydroxyprogesterone Caproate API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Hydroxyprogesterone Caproate manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Hydroxyprogesterone Caproate supplier is an individual or a company that provides Hydroxyprogesterone Caproate active pharmaceutical ingredient (API) or Hydroxyprogesterone Caproate finished formulations upon request. The Hydroxyprogesterone Caproate suppliers may include Hydroxyprogesterone Caproate API manufacturers, exporters, distributors and traders.
click here to find a list of Hydroxyprogesterone Caproate suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Hydroxyprogesterone Caproate DMF (Drug Master File) is a document detailing the whole manufacturing process of Hydroxyprogesterone Caproate active pharmaceutical ingredient (API) in detail. Different forms of Hydroxyprogesterone Caproate DMFs exist exist since differing nations have different regulations, such as Hydroxyprogesterone Caproate USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Hydroxyprogesterone Caproate DMF submitted to regulatory agencies in the US is known as a USDMF. Hydroxyprogesterone Caproate USDMF includes data on Hydroxyprogesterone Caproate's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Hydroxyprogesterone Caproate USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Hydroxyprogesterone Caproate suppliers with USDMF on PharmaCompass.
A Hydroxyprogesterone Caproate written confirmation (Hydroxyprogesterone Caproate WC) is an official document issued by a regulatory agency to a Hydroxyprogesterone Caproate manufacturer, verifying that the manufacturing facility of a Hydroxyprogesterone Caproate active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Hydroxyprogesterone Caproate APIs or Hydroxyprogesterone Caproate finished pharmaceutical products to another nation, regulatory agencies frequently require a Hydroxyprogesterone Caproate WC (written confirmation) as part of the regulatory process.
click here to find a list of Hydroxyprogesterone Caproate suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Hydroxyprogesterone Caproate as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Hydroxyprogesterone Caproate API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Hydroxyprogesterone Caproate as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Hydroxyprogesterone Caproate and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Hydroxyprogesterone Caproate NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Hydroxyprogesterone Caproate suppliers with NDC on PharmaCompass.
Hydroxyprogesterone Caproate Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Hydroxyprogesterone Caproate GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Hydroxyprogesterone Caproate GMP manufacturer or Hydroxyprogesterone Caproate GMP API supplier for your needs.
A Hydroxyprogesterone Caproate CoA (Certificate of Analysis) is a formal document that attests to Hydroxyprogesterone Caproate's compliance with Hydroxyprogesterone Caproate specifications and serves as a tool for batch-level quality control.
Hydroxyprogesterone Caproate CoA mostly includes findings from lab analyses of a specific batch. For each Hydroxyprogesterone Caproate CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Hydroxyprogesterone Caproate may be tested according to a variety of international standards, such as European Pharmacopoeia (Hydroxyprogesterone Caproate EP), Hydroxyprogesterone Caproate JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Hydroxyprogesterone Caproate USP).

