



API Suppliers

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
0
Listed Suppliers
0
0

USA (Orange Book)

Europe
0

Canada
0

Australia
0

South Africa
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
USP
0
JP
0
Other Listed Suppliers
0
0
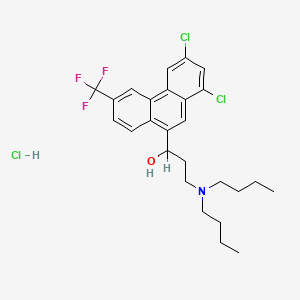

1. 1-(1,3-dichloro-6-trifluoromethyl-9-phenanthryl)-3-di(n-butyl)aminopropanol Hcl
2. Halfan
3. Halofantrine
4. Wr 171,699
5. Wr 171669
6. Wr-171,669
7. Wr-171699
1. 36167-63-2
2. Halfan
3. Halofantrine Hcl
4. Halofantrine Hydrochloride [usan]
5. Wr-171669
6. Skf-102886
7. Halofantrine (hydrochloride)
8. 3-(dibutylamino)-1-[1,3-dichloro-6-(trifluoromethyl)phenanthren-9-yl]propan-1-ol;hydrochloride
9. (-)-halofantrine Hydrochloride
10. 2b7enl644k
11. 106927-11-1
12. Halofantrine Hydrochloride, (-)-
13. H77dl0y630
14. 3-(dibutylamino)-1-(1,3-dichloro-6-(trifluoromethyl)phenanthren-9-yl)propan-1-ol Hydrochloride
15. 9-(3-(dibutylamino)-1-hydroxypropyl)-1,3-dichloro-6-(trifluoromethyl)phenanthrene Hydrochloride
16. Dl-wr 171669
17. Ncgc00016833-01
18. Skf-102886;wr-171669
19. Dsstox_cid_25464
20. Dsstox_rid_80895
21. Dsstox_gsid_45464
22. Halofantrine Hydrochloride (usan)
23. Halofantrino
24. Halofantrino [spanish]
25. Mls002154111
26. 66051-64-7
27. Cas-36167-63-2
28. (+-)-halofantrine Hydrochloride
29. Wr 171669
30. Wr-171,669
31. Smr001233418
32. Einecs 252-895-4
33. Unii-h77dl0y630
34. Halfan (tn)
35. Mfcd00879136
36. Skf 102886
37. Halofantrine Hydrochlorid
38. 1,3-dichloro-6-trifluoromethyl-9-(3-(dibutylamino)-1-hydroxypropyl)phenanthrene Hcl
39. 1-(1,3-dichloro-6-trifluoromethyl-9-phenanthryl)-3-(di-n-butylamino)propanol Hydrochloride
40. Unii-2b7enl644k
41. 1,3-dichloro-alpha-(2-(dibutylamino)ethyl)-6-(trifluoromethyl)-9-phenanthrenemethanol Hydrochloride
42. Schembl466895
43. Chembl1200901
44. Dtxsid0045464
45. Hy-a0148a
46. Hms1571o03
47. Tox21_110637
48. Akos030254762
49. Tox21_110637_1
50. Ccg-221031
51. Halofantrine Hydrochloride [mi]
52. Sk&f-102866
53. Ncgc00179250-03
54. (+/-)-halofantrine Hydrochloride
55. 1,3-dichloro-alpha-(2-(dibutylamino)ethyl)-6-(trifluoromethyl)phenanthren-1-methanol Hydrochloride
56. 9-phenanthrenemethanol, 1,3-dichloro-alpha-(2-(dibutylamino)ethyl)-6-(trifluoromethyl)-, Hydrochloride
57. Halofantrine Hydrochloride [mart.]
58. Halofantrine Hydrochloride [vandf]
59. Halofantrine Hydrochloride [who-dd]
60. Cs-0031225
61. Ft-0600399
62. D02485
63. Halofantrine Hydrochloride [ep Impurity]
64. Halofantrine Hydrochloride [orange Book]
65. Halofantrine Hydrochloride [ep Monograph]
66. 167h632
67. Halofantrine Hydrochloride, >=98% (hplc), Solid
68. Sr-01000841216
69. Sr-01000841216-2
70. W-106636
71. Halofantrine Hydrochloride, European Pharmacopoeia (ep) Reference Standard
72. 1-[1,3-dichloro-6-trifluoromethyl-9-phenanthryl]-3-di(n-butyl)aminopropanol Hydrochloride
73. 1,3-dichloro-.alpha.-(2-(dibutylamino)ethyl)-6-(trifluoromethyl)-9-phenanthrenemethanol Hydrochloride
74. 1,3-dichloro-alpha-[2-(dibutylamino)ethyl]-6-(trifluoromethyl)-9-phenanthrenemethanol;(+/-)-halofantrine Hydrochloride
75. 9-phenanthrenemethanol, 1,3-dichloro-.alpha.-(2-(dibutylamino)ethyl)-6-(trifluoromethyl)-, Hydrochloride
76. 9-phenanthrenemethanol, 1,3-dichloro-.alpha.-(2-(dibutylamino)ethyl)-6-(trifluoromethyl)-, Hydrochloride (1:1)
77. 9-phenanthrenemethanol, 1,3-dichloro-.alpha.-(2-(dibutylamino)ethyl)-6-(trifluoromethyl)-, Hydrochloride, (-)-
78. 9-phenanthrenemethanol, 1,3-dichloro-alpha-(2-(dibutylamino)ethyl)-6-(trifluoromethyl)-, Hydrochloride, (-)-
| Molecular Weight | 536.9 g/mol |
|---|---|
| Molecular Formula | C26H31Cl3F3NO |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 10 |
| Exact Mass | 535.142332 g/mol |
| Monoisotopic Mass | 535.142332 g/mol |
| Topological Polar Surface Area | 23.5 Ų |
| Heavy Atom Count | 34 |
| Formal Charge | 0 |
| Complexity | 584 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Antimalarials
Agents used in the treatment of malaria. They are usually classified on the basis of their action against plasmodia at different stages in their life cycle in the human. (From AMA, Drug Evaluations Annual, 1992, p1585) (See all compounds classified as Antimalarials.)


