
Synopsis
Synopsis
0
VMF
0
FDA Orange Book
DRUG PRODUCT COMPOSITIONS
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
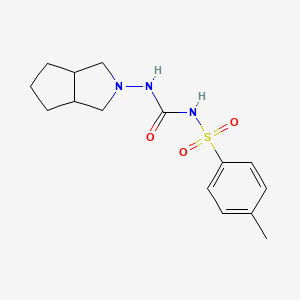

1. Diabrezide
2. Diaglyk
3. Diaikron
4. Diamicron
5. Gen Gliclazide
6. Gen-gliclazide
7. Gliklazid
8. Glyade
9. Glyclazide
10. Novo Gliclazide
11. Novo-gliclazide
12. S 1702
13. S 852
14. S-1702
15. S-852
16. S1702
17. S852
1. 21187-98-4
2. Glimicron
3. 1-(3,3a,4,5,6,6a-hexahydro-1h-cyclopenta[c]pyrrol-2-yl)-3-(4-methylphenyl)sulfonylurea
4. 1-(3-azabicyclo(3.3.0)oct-3-yl)-3-(p-tolylsulfonyl)urea
5. Chebi:31654
6. 1-(hexahydrocyclopenta(c)pyrrol-2(1h)-yl)-3-(p-tolylsulfonyl)urea
7. Gliclazidum [inn-latin]
8. Gliclazida [inn-spanish]
9. Gliclazida
10. N-(hexahydrocyclopenta[c]pyrrol-2(1h)-ylcarbamoyl)-4-methylbenzenesulfonamide
11. 1-[(4-methylbenzene)sulfonyl]-3-{octahydrocyclopenta[c]pyrrol-2-yl}urea
12. Nsc-758673
13. Se-1702
14. S1702;se1702
15. Dsstox_cid_3095
16. N-((hexahydrocyclopenta[c]pyrrol-2(1h)-yl)carbamoyl)-4-methylbenzenesulfonamide
17. Dsstox_rid_76872
18. Dsstox_gsid_23095
19. S-852
20. S-1702
21. N-(4-methylbenzenesulfonyl)-n'-(3-azabicyclo(3.3.0)oct-3-yl)urea
22. Glimicron (tn)
23. Smr000542971
24. Sr-01000816184
25. 1-(3-azabicyclo[3.3.0]oct-3-yl)-3-p-tolylsulphonylurea
26. Mfcd00409893
27. J3.151h
28. Gliclazide,(s)
29. Benzenesulfonamide, N-[[(hexahydrocyclopenta[c]pyrrol-2(1h)-yl)amino]carbonyl]-4-methyl-
30. Ncgc00016751-01
31. Prestwick_869
32. Cas-21187-98-4
33. Gliclazide (diamicron)
34. Spectrum_001478
35. Specplus_000870
36. Prestwick0_000558
37. Prestwick1_000558
38. Prestwick2_000558
39. Prestwick3_000558
40. Spectrum3_001862
41. Spectrum4_000598
42. Spectrum5_000753
43. Gliclazide (jp17/inn)
44. Schembl16387
45. Bspbio_000635
46. Bspbio_003304
47. Kbiogr_001096
48. Kbioss_001958
49. Mls001215197
50. Mls001304077
51. Mls001304118
52. Divk1c_006966
53. Gliclazide, Powder, >=98%
54. Spectrum1504145
55. Spectrum1505013
56. Spbio_002556
57. Bpbio1_000699
58. Chembl427216
59. Dtxsid9023095
60. Urea, 1-(hexahydrocyclopenta[c]pyrrol-2(1h)-yl)-3-(p-tolylsulfonyl)-
61. Kbio1_001910
62. Kbio2_001958
63. Kbio2_004526
64. Kbio2_007094
65. Kbio3_002806
66. 1-[3-azabicyclo[3.3.0]oct-3-yl]-3-p-toluenesulfonylurea
67. Hms1569p17
68. Hms1922d15
69. Hms2090k16
70. Hms2096p17
71. Hms2855p09
72. Hms3656c22
73. Hms3713p17
74. Hms3744o13
75. Pharmakon1600-01504145
76. Bcp21240
77. Hy-b0753
78. Tox21_110590
79. Bbl012275
80. Bdbm50103512
81. Nsc758673
82. Nsc813216
83. S2601
84. Se1702
85. Stk803142
86. Akos003237903
87. Akos016340698
88. Tox21_110590_1
89. Ab05958
90. Ccg-213918
91. Db01120
92. Ks-1067
93. Nsc-813216
94. N-[(hexahydrocyclopenta[c]pyrrol-2(1h)-ylamino)carbonyl]-4-methylbenzenesulfonamide
95. Ncgc00095107-01
96. Ncgc00095107-02
97. Ncgc00095107-03
98. Ncgc00095107-04
99. Ncgc00095107-05
100. Ncgc00095107-06
101. Ncgc00095107-09
102. Ncgc00095107-10
103. Ac-12045
104. Sbi-0052662.p002
105. Ab00053165
106. Ft-0626712
107. G0381
108. Sw196994-3
109. D01599
110. D83168
111. Ab00053165-09
112. Ab00053165_10
113. Ab00053165_11
114. 187g984
115. A815188
116. Q290001
117. J-013905
118. J-522753
119. Sr-01000816184-2
120. Sr-01000816184-3
121. Sr-01000816184-4
122. Brd-a61154809-001-04-3
123. Gliclazide, British Pharmacopoeia (bp) Reference Standard
124. Gliclazide, European Pharmacopoeia (ep) Reference Standard
125. 1-(4-methylbenzenesulfonyl)-3-{octahydrocyclopenta[c]pyrrol-2-yl}urea
126. 1-(3,3a,4,5,6,6a-hexahydro-1h-cyclopenta[c]pyrrol-2-yl)-3-(4-methylphenyl)sulfonyl-urea
127. Benzenesulfonamide,n-[[(hexahydrocyclopenta[c]pyrrol-2(1h)-yl)amino]carbonyl]-4-methyl-
128. Benzenesulfonamide, N-[[(hexahydrocyclopenta[c]pyrrol-2(1h)-yl)amino]carbonyl]-4-methyl
| Molecular Weight | 323.4 g/mol |
|---|---|
| Molecular Formula | C15H21N3O3S |
| XLogP3 | 1.5 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 3 |
| Exact Mass | 323.13036271 g/mol |
| Monoisotopic Mass | 323.13036271 g/mol |
| Topological Polar Surface Area | 86.9 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 497 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 2 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For the treatment of NIDDM in conjunction with diet and exercise.
Based on the pharmacological properties, gliclazide is a second generation sulphonylurea which acts as a hypoglycemic agent. It stimulates β cells of the islet of Langerhans in the pancreas to release insulin. It also enhances peripheral insulin sensitivity. Overall, it potentiates insulin release and improves insulin dynamics.
Hypoglycemic Agents
Substances which lower blood glucose levels. (See all compounds classified as Hypoglycemic Agents.)
A10BB09
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
A - Alimentary tract and metabolism
A10 - Drugs used in diabetes
A10B - Blood glucose lowering drugs, excl. insulins
A10BB - Sulfonylureas
A10BB09 - Gliclazide
Absorption
Rapidly and well absorbed but may have wide inter- and intra-individual variability. Peak plasma concentrations occur within 4-6 hours of oral administration.
Route of Elimination
Metabolites and conjugates are eliminated primarily by the kidneys (60-70%) and also in the feces (10-20%).
Extensively metabolized in the liver. Less than 1% of the orally administered dose appears unchanged in the urine. Metabolites include oxidized and hydroxylated derivates, as well as glucuronic acid conjugates.
Gliclazide has known human metabolites that include 6-hydroxy-gliclazide, 7-hydroxy-gliclazide, and Methylhydroxygliclazide.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
10.4 hours. Duration of action is 10-24 hours.
Gliclazide binds to the β cell sulfonyl urea receptor (SUR1). This binding subsequently blocks the ATP sensitive potassium channels. The binding results in closure of the channels and leads to a resulting decrease in potassium efflux leads to depolarization of the β cells. This opens voltage-dependent calcium channels in the β cell resulting in calmodulin activation, which in turn leads to exocytosis of insulin containing secretorty granules.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
54
PharmaCompass offers a list of Gliclazide API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Gliclazide manufacturer or Gliclazide supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Gliclazide manufacturer or Gliclazide supplier.
PharmaCompass also assists you with knowing the Gliclazide API Price utilized in the formulation of products. Gliclazide API Price is not always fixed or binding as the Gliclazide Price is obtained through a variety of data sources. The Gliclazide Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Gliclazide manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Gliclazide, including repackagers and relabelers. The FDA regulates Gliclazide manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Gliclazide API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Gliclazide manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Gliclazide supplier is an individual or a company that provides Gliclazide active pharmaceutical ingredient (API) or Gliclazide finished formulations upon request. The Gliclazide suppliers may include Gliclazide API manufacturers, exporters, distributors and traders.
click here to find a list of Gliclazide suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Gliclazide DMF (Drug Master File) is a document detailing the whole manufacturing process of Gliclazide active pharmaceutical ingredient (API) in detail. Different forms of Gliclazide DMFs exist exist since differing nations have different regulations, such as Gliclazide USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Gliclazide DMF submitted to regulatory agencies in the US is known as a USDMF. Gliclazide USDMF includes data on Gliclazide's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Gliclazide USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Gliclazide suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Gliclazide Drug Master File in Japan (Gliclazide JDMF) empowers Gliclazide API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Gliclazide JDMF during the approval evaluation for pharmaceutical products. At the time of Gliclazide JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Gliclazide suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Gliclazide Drug Master File in Korea (Gliclazide KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Gliclazide. The MFDS reviews the Gliclazide KDMF as part of the drug registration process and uses the information provided in the Gliclazide KDMF to evaluate the safety and efficacy of the drug.
After submitting a Gliclazide KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Gliclazide API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Gliclazide suppliers with KDMF on PharmaCompass.
A Gliclazide CEP of the European Pharmacopoeia monograph is often referred to as a Gliclazide Certificate of Suitability (COS). The purpose of a Gliclazide CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Gliclazide EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Gliclazide to their clients by showing that a Gliclazide CEP has been issued for it. The manufacturer submits a Gliclazide CEP (COS) as part of the market authorization procedure, and it takes on the role of a Gliclazide CEP holder for the record. Additionally, the data presented in the Gliclazide CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Gliclazide DMF.
A Gliclazide CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Gliclazide CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Gliclazide suppliers with CEP (COS) on PharmaCompass.
A Gliclazide written confirmation (Gliclazide WC) is an official document issued by a regulatory agency to a Gliclazide manufacturer, verifying that the manufacturing facility of a Gliclazide active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Gliclazide APIs or Gliclazide finished pharmaceutical products to another nation, regulatory agencies frequently require a Gliclazide WC (written confirmation) as part of the regulatory process.
click here to find a list of Gliclazide suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Gliclazide as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Gliclazide API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Gliclazide as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Gliclazide and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Gliclazide NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Gliclazide suppliers with NDC on PharmaCompass.
Gliclazide Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Gliclazide GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Gliclazide GMP manufacturer or Gliclazide GMP API supplier for your needs.
A Gliclazide CoA (Certificate of Analysis) is a formal document that attests to Gliclazide's compliance with Gliclazide specifications and serves as a tool for batch-level quality control.
Gliclazide CoA mostly includes findings from lab analyses of a specific batch. For each Gliclazide CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Gliclazide may be tested according to a variety of international standards, such as European Pharmacopoeia (Gliclazide EP), Gliclazide JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Gliclazide USP).

