



API Suppliers

US DMFs Filed

CEP/COS Certifications

JDMFs Filed
Other Certificates
Other Suppliers

USA (Orange Book)

Europe

Canada

Australia

South Africa
Uploaded Dossiers
U.S. Medicaid
Annual Reports
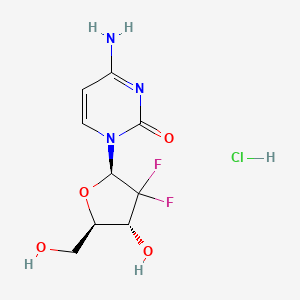

1. 188011, Ly
2. 2' Deoxy 2' Difluorocytidine
3. 2' Deoxy 2',2'' Difluorocytidine 5' O Monophosphate
4. 2',2'-dfdc
5. 2',2'-difluoro-2'-deoxycytidine
6. 2',2'-difluorodeoxycytidine
7. 2'-deoxy-2',2''-difluorocytidine-5'-o-monophosphate
8. 2'-deoxy-2'-difluorocytidine
9. Dfdcyd
10. Gemcitabine
11. Gemcitabine, (alpha-d-threo-pentofuranosyl)-isomer
12. Gemcitabine, (beta-d-threo-pentafuranosyl)-isomer
13. Gemcitabine, (d-threo-pentafuranosyl)-isomer
14. Gemicitabine
15. Gemzar
16. Hydrochloride, Gemcitabine
17. Ly 188011
18. Ly-188011
1. Gemcitabine Hcl
2. 122111-03-9
3. Gemzar
4. Ly188011 Hydrochloride
5. Gemcitabine (hydrochloride)
6. 2'-deoxy-2',2'-difluorocytidine Monohydrochloride
7. Cytidine, 2'-deoxy-2',2'-difluoro-, Monohydrochloride
8. Dfdc
9. 4-amino-1-((2r,4r,5r)-3,3-difluoro-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)pyrimidin-2(1h)-one Hydrochloride
10. Gemcitabine (as Hydrochloride)
11. U347pv74il
12. Chebi:31647
13. 2',2'-difluoro-2'-deoxycytidine Hydrochloride
14. Ly-188011 Hydrochloride
15. Ly-188011
16. 122111-03-9 (hcl)
17. Dsstox_cid_27827
18. Dsstox_rid_82590
19. Dsstox_gsid_47849
20. 2'-deoxy-2',2'-difluorocytidine Hydrochloride
21. 4-amino-1-[(2r,4r,5r)-3,3-difluoro-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]pyrimidin-2-one;hydrochloride
22. Dizirconium Silicide
23. Cas-122111-03-9
24. Gemcitabine Hydrochloride [usan]
25. Gemcitabine Hcl (gemzar)
26. Ly 188011
27. Unii-u347pv74il
28. Mfcd01735988
29. Ncgc00168784-01
30. Gemzar (tn)
31. Infugem
32. Gemcitabine Hydrochloride [usan:usp]
33. Inno-d07001
34. Schembl4366
35. Chembl1637
36. Ly 188011 Hydrochloride
37. Mls003915643
38. Gemcitabine Hydrochloride,(s)
39. Dtxsid3047849
40. Ex-a837
41. 2'-deoxy-2',2'-difluorocytidine Monohydrochloride (beta-isomer)
42. Act06731
43. Hy-b0003
44. Ly-188011 . Hcl
45. Tox21_112644
46. Bdbm50247964
47. Gemcitabine Hydrochloride (jan/usp)
48. S1149
49. Gemcitabine Hydrochloride [mi]
50. Akos015999730
51. Tox21_112644_1
52. Ccg-267459
53. Cs-0755
54. Gemcitabine Hcl (gemzar,ly188011)
55. Gemcitabine Hydrochloride [jan]
56. 2'-deoxy-2',2'-difluorocytidine Hcl
57. Gemcitabine Hydrochloride [mart.]
58. Gemcitabine Hydrochloride [vandf]
59. Ncgc00168784-03
60. As-13656
61. Bg164501
62. Gemcitabine Hydrochloride [usp-rs]
63. Gemcitabine Hydrochloride [who-dd]
64. Smr000469146
65. Gemcitabine Hydrochloride, >=98% (hplc)
66. Gemcitabine Hydrochloride,gemzar, Ly-188011
67. D01155
68. Gemcitabine Hydrochloride [ep Monograph]
69. Gemcitabine Hydrochloride [orange Book]
70. 2'-deoxy-2',2'-difluorocytidine, Hydrochloride
71. Gemcitabine Hydrochloride [usp Monograph]
72. 111g039
73. W-60402
74. (+)-2'-deoxy-2',2'-difluorocytidine Hydrochloride
75. J-700175
76. Q27114559
77. 2'-deoxy-2',2'-difluorocytidine Monohydrochloride (.beta.-isomer)
78. Gemcitabine Hydrochloride, European Pharmacopoeia (ep) Reference Standard
79. Gemcitabine Hydrochloride, United States Pharmacopeia (usp) Reference Standard
80. (2'r,4'r,5'r)-4-amino-1-(3,3-difluoro-4-hydroxy-5-hydroxymethyl-tetrahydro-furan-2-yl)-1h-pyridin-2-one Hydrochloride
81. 4-amino-1-((2r,4r,5r)-3,3-difluoro-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl)pyrimidin-2-one Hydrochloride
82. 4-amino-1-((2r,4r,5r)-3,3-difluoro-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)pyrimidin-2(1h)-onehydrochloride
1. Gemcitabine Free Base
| Molecular Weight | 299.66 g/mol |
|---|---|
| Molecular Formula | C9H12ClF2N3O4 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 2 |
| Exact Mass | 299.0484399 g/mol |
| Monoisotopic Mass | 299.0484399 g/mol |
| Topological Polar Surface Area | 108 Ų |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 426 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 4 | |
|---|---|
| Drug Name | Gemcitabine hydrochloride |
| Drug Label | Gemzar (gemcitabine for injection, USP) is a nucleoside metabolic inhibitor that exhibits antitumor activity. Gemcitabine HCl is 2-deoxy-2,2-difluorocytidine monohydrochloride (-isomer). The structural formula is as follows: The empirical... |
| Active Ingredient | Gemcitabine hydrochloride |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 2gm base/vial; eq 200mg base/vial; 1gm/26.3ml (38mg/ml); 2gm/52.6ml (38mg/ml); 200mg/5.26ml (38mg/ml); eq 1gm base/vial |
| Market Status | Prescription |
| Company | Hospira; Jiangsu Hansoh Pharm; Fresenius Kabi Oncol; Accord Hlthcare; Cipla; Sun Pharma Global; Actavis Elizabeth; Emcure Pharms; Teva Pharms; Fresenius Kabi Usa; Onco Therapies; Hameln Rds Gmbh; Luitpold; Sagent Pharms; Dr Reddys Labs |
| 2 of 4 | |
|---|---|
| Drug Name | Gemzar |
| PubMed Health | Gemcitabine (Injection) |
| Drug Classes | Antineoplastic Agent |
| Drug Label | Gemzar (gemcitabine for injection, USP) is a nucleoside metabolic inhibitor that exhibits antitumor activity. Gemcitabine HCl is 2-deoxy-2,2-difluorocytidine monohydrochloride (-isomer). The structural formula is as follows: The empirical... |
| Active Ingredient | Gemcitabine hydrochloride |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 200mg base/vial; eq 1gm base/vial |
| Market Status | Prescription |
| Company | Lilly |
| 3 of 4 | |
|---|---|
| Drug Name | Gemcitabine hydrochloride |
| Drug Label | Gemzar (gemcitabine for injection, USP) is a nucleoside metabolic inhibitor that exhibits antitumor activity. Gemcitabine HCl is 2-deoxy-2,2-difluorocytidine monohydrochloride (-isomer). The structural formula is as follows: The empirical... |
| Active Ingredient | Gemcitabine hydrochloride |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 2gm base/vial; eq 200mg base/vial; 1gm/26.3ml (38mg/ml); 2gm/52.6ml (38mg/ml); 200mg/5.26ml (38mg/ml); eq 1gm base/vial |
| Market Status | Prescription |
| Company | Hospira; Jiangsu Hansoh Pharm; Fresenius Kabi Oncol; Accord Hlthcare; Cipla; Sun Pharma Global; Actavis Elizabeth; Emcure Pharms; Teva Pharms; Fresenius Kabi Usa; Onco Therapies; Hameln Rds Gmbh; Luitpold; Sagent Pharms; Dr Reddys Labs |
| 4 of 4 | |
|---|---|
| Drug Name | Gemzar |
| PubMed Health | Gemcitabine (Injection) |
| Drug Classes | Antineoplastic Agent |
| Drug Label | Gemzar (gemcitabine for injection, USP) is a nucleoside metabolic inhibitor that exhibits antitumor activity. Gemcitabine HCl is 2-deoxy-2,2-difluorocytidine monohydrochloride (-isomer). The structural formula is as follows: The empirical... |
| Active Ingredient | Gemcitabine hydrochloride |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 200mg base/vial; eq 1gm base/vial |
| Market Status | Prescription |
| Company | Lilly |
Treatment of urothelial carcinoma
Antimetabolites, Antineoplastic
Antimetabolites that are useful in cancer chemotherapy. (See all compounds classified as Antimetabolites, Antineoplastic.)




