



API Suppliers

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
0
Listed Suppliers
0
0

USA (Orange Book)
0

Europe
0

Canada

Australia
0

South Africa
0
Uploaded Dossiers
U.S. Medicaid
0
Annual Reports
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
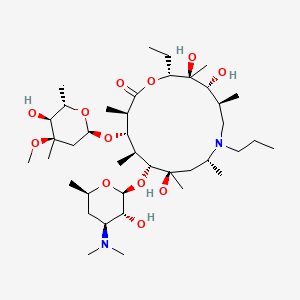

1. Zactran
1. 145435-72-9
2. Ml-1709460
3. Ml-1,709,460
4. Ml-460
5. Ze856183s0
6. (2r,3s,4r,5s,8r,10r,11r,12s,13s,14r)-11-(((2s,3r,4s,6r)-4-(dimethylamino)-3-hydroxy-6-methyltetrahydro-2h-pyran-2-yl)oxy)-2-ethyl-3,4,10-trihydroxy-13-(((2r,4r,5s,6s)-5-hydroxy-4-methoxy-4,6-dimethyltetrahydro-2h-pyran-2-yl)oxy)-3,5,8,10,12,14-hexamethyl-7-propyl-1-oxa-7-azacyclopentadecan-15-one
7. (2r,3s,4r,5s,8r,10r,11r,12s,13s,14r)-11-[(2s,3r,4s,6r)-4-(dimethylamino)-3-hydroxy-6-methyloxan-2-yl]oxy-2-ethyl-3,4,10-trihydroxy-13-[(2r,4r,5s,6s)-5-hydroxy-4-methoxy-4,6-dimethyloxan-2-yl]oxy-3,5,8,10,12,14-hexamethyl-7-propyl-1-oxa-7-azacyclopentadecan-15-one
8. Gamithromycin [usan:inn]
9. Unii-ze856183s0
10. (2r,3s,4r,5s,8r,10r,11r,12s,13s,14r)-11-[(2s,3r,4s,6r)-4-(dimethylamino)-3-hydroxy-6-methyloxan-2-yl]oxy-2-ethyl-3,4,10-trihydroxy-13-[(2r,4r,5s,6s)-5-hydroxy-4-methoxy-4,6-dimethyloxan-2-yl]oxy-3,5,8
11. Gamithromycin [mi]
12. Gamithromycin [inn]
13. Gamithromycin [usan]
14. Chembl2107342
15. Dtxsid90904260
16. Gamithromycin [green Book]
17. Ex-a1349
18. Mfcd09954125
19. S5328
20. Zinc169292837
21. Cs-7858
22. Db11416
23. Ds-4441
24. Gamithromycin 100 Microg/ml In Methanol
25. Ac-32611
26. Gamithromycin [ema Epar Veterinary]
27. Hy-108365
28. C72760
29. 435g729
30. A884620
31. J-008105
32. (2r,3s,4r,5s,8r,10r,11r,12s,13s,14r)-13-((2,6-dideoxy-3-c-methyl-3-o-methyl-.alpha.-l-ribo-hexopyranosyl)oxy)-2-ethyl-3,4,10-trihydroxy-3,5,8,10,12,14-hexamethyl-7-propyl-11-((3,4,6-trideoxy-3-(dimethylamino)-.beta.-d-xylo-hexopyranosyl)oxy)-1-oxa-7-azacyclopentadecan-15-one
33. (2r,3s,4r,5s,8r,10r,11r,12s,13s,14r)-13-[(2,6-dideoxy-3-c-methyl-3-o-methyl-alpha-l-ribo-hexopyranosyl)
34. (2r,3s,4r,5s,8r,10r,11r,12s,13s,14r)-13-[(2,6-dideoxy-3-cmethyl-3-o-methyl-a-l-ribo-hexopyranosyl)oxy]-2-ethyl-3,4,10-trihydroxy-3,5,8,10,12,14-hexamethyl-7-propyl-11-[[3,4,6-trideoxy-3-(dimethylamino)-b-d-xylo-hexopyranosyl]oxy]-1-oxa-7-azacyclopentadecan-15-one
35. 1-oxa-7-azacyclopentadecan-15-one, 13-((2,6-dideoxy-3-c-methyl-3-o-methyl-.alpha.-l-ribo-hexopyranosyl)oxy)-2-ethyl-3,4,10-trihydroxy-3,5,8,10,12,14-hexamethyl-7-propyl-11-((3,4,6-trideoxy-3-(dimethylamino)-.beta.-d-xylo-hexopyranosyl)oxy)-, (2r,3s,4r,5s,8r,10r,11r,12s,13s,14r)-
36. 1-oxa-7-azacyclopentadecan-15-one,13-[(2,6-dideoxy-3-c-methyl-3-o-methyl-a-l-ribo-hexopyranosyl)oxy]-2-ethyl-3,4,10-trihydroxy-3,5,8,10,12,14-hexamethyl-7-propyl-11-[[3,4,6-trideoxy-3-(dimethylamino)-b-d-xylo-hexopyranosyl]oxy]-,[2r-(2r*,3s*,4r*,5s*,8r*,10r*,11r*,12s*,13s*,14r*)]-
| Molecular Weight | 777.0 g/mol |
|---|---|
| Molecular Formula | C40H76N2O12 |
| XLogP3 | 4.9 |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 14 |
| Rotatable Bond Count | 9 |
| Exact Mass | 776.53982586 g/mol |
| Monoisotopic Mass | 776.53982586 g/mol |
| Topological Polar Surface Area | 180 Ų |
| Heavy Atom Count | 54 |
| Formal Charge | 0 |
| Complexity | 1180 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 18 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
* Cattle:
Treatment and metaphylaxis of bovine respiratory disease (BRD) associated with Mannheimia haemolytica, Pasteurella multocida and Histophilus somni. The presence of the disease in the herd should be established before metaphylactic use.
* Pigs:
Treatment of swine respiratory disease (SRD) associated with Actinobacillus pleuropneumoniae, Pasteurella multocida, Haemophilus parasuis and Bordetella bronchiseptica.
* Sheep:
Treatment of infectious pododermatitis (foot rot) associated with virulent Dichelobacter nodosus and Fusobacterium necrophorum requiring systemic treatment.
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
QJ01FA95


