


API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
0
Listed Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
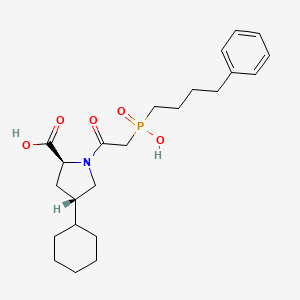

1. Fosfenopril
2. Fosinoprilic Acid
3. Sq 27519
4. Sq-27,519
5. Sq-27519
1. Fosinopril Diacid
2. 95399-71-6
3. Fosinoprilic Acid
4. Fosfenopril
5. Fosinoprilatum
6. Sq 27,519
7. Sq-27519
8. (4s)-4-cyclohexyl-1-((hydroxy(4-phenylbutyl)phosphinyl)acetyl)-l-proline
9. Chembl581
10. Fosinopril Related Compound A
11. S312ey6zt8
12. Chebi:116962
13. So-27519
14. L-proline, 4-cyclohexyl-1-((hydroxy(4-phenylbutyl)phosphinyl)acetyl)-, Trans-
15. 4-cyclohexyl-1-{2-[hydroxy-(4-phenyl-butyl)-phosphinoyl]-acetyl}-pyrrolidine-2-carboxylic Acid
16. (2s,4s)-4-cyclohexyl-1-(2-(hydroxy(4-phenylbutyl)phosphoryl)acetyl)pyrrolidine-2-carboxylic Acid
17. (2s,4s)-4-cyclohexyl-1-[2-[hydroxy(4-phenylbutyl)phosphoryl]acetyl]pyrrolidine-2-carboxylic Acid
18. (4s)-4-cyclohexyl-[(4-phenylbutyl)phosphinyl]acetyl-l-proline
19. (4s)-4-cyclohexyl-1-{[hydroxy(4-phenylbutyl)phosphoryl]acetyl}-l-proline
20. L-proline, 4-cyclohexyl-1-[[hydroxy(4-phenylbutyl)phosphinyl]acetyl]-,(4s)-
21. Fosinoprilat [usan:inn]
22. Fosinoprilatum [inn-latin]
23. (2s,4s)-4-cyclohexyl-1-{2-[hydroxy(4-phenylbutyl)phosphoryl]acetyl}pyrrolidine-2-carboxylic Acid
24. (2s,4s)-4-cyclohexyl-1-{2-[hydroxy-(4-phenyl-butyl)-phosphinoyl]-acetyl}-pyrrolidine-2-carboxylic Acid
25. Unii-s312ey6zt8
26. Forsinoprilat
27. Ks8
28. Fosinopril Impurity A
29. Sq-27,519
30. Pu6ba2d2md
31. Fosinoprilat (usan/inn)
32. Fosinoprilat [inn]
33. Fosinoprilat [usan]
34. Schembl124537
35. Fosinopril Diacid [mi]
36. Gtpl6457
37. Dtxsid80869253
38. Zinc4213382
39. Bdbm50018849
40. Db14207
41. Sq27,519
42. Hy-107352
43. So 27,519
44. Cs-0028200
45. C21542
46. D03772
47. Fosinopril Related Compound A [usp-rs]
48. Fosinopril Sodium Impurity A [ep Impurity]
49. Fosinopril Related Compound A [usp Impurity]
50. Q27077720
51. 1[{hydroxy(4-phenylbutyl)phosphinyl}acetyl]-(trans)-4-cyclohexyl-l-proline
52. Trans-4-cyclohexyl-1-[[hydroxy(4-phenylbutyl)phosphinyl]acetyl]-l-proline
53. L-proline,4-cyclohexyl-1-[2-[hydroxy(4-phenylbutyl)phosphinyl]acetyl]-,(4s)-
54. (2s,4s)-4-cyclohexyl-1-((hydroxy(4-phenylbutyl)phosphoryl)acetyl)pyrrolidine-2-carboxylic Acid
55. (2s,4s)-4-cyclohexyl-1-[2-(hydroxy-(4-phenylbutyl)phosphoryl)acetyl]pyrrolidine-2-carboxylic Acid
| Molecular Weight | 435.5 g/mol |
|---|---|
| Molecular Formula | C23H34NO5P |
| XLogP3 | 3.8 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 9 |
| Exact Mass | 435.21746018 g/mol |
| Monoisotopic Mass | 435.21746018 g/mol |
| Topological Polar Surface Area | 94.9 Ų |
| Heavy Atom Count | 30 |
| Formal Charge | 0 |
| Complexity | 627 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Angiotensin-Converting Enzyme Inhibitors
A class of drugs whose main indications are the treatment of hypertension and heart failure. They exert their hemodynamic effect mainly by inhibiting the renin-angiotensin system. They also modulate sympathetic nervous system activity and increase prostaglandin synthesis. They cause mainly vasodilation and mild natriuresis without affecting heart rate and contractility. (See all compounds classified as Angiotensin-Converting Enzyme Inhibitors.)
Antihypertensive Agents
Drugs used in the treatment of acute or chronic vascular HYPERTENSION regardless of pharmacological mechanism. Among the antihypertensive agents are DIURETICS; (especially DIURETICS, THIAZIDE); ADRENERGIC BETA-ANTAGONISTS; ADRENERGIC ALPHA-ANTAGONISTS; ANGIOTENSIN-CONVERTING ENZYME INHIBITORS; CALCIUM CHANNEL BLOCKERS; GANGLIONIC BLOCKERS; and VASODILATOR AGENTS. (See all compounds classified as Antihypertensive Agents.)
Anti-Arrhythmia Agents
Agents used for the treatment or prevention of cardiac arrhythmias. They may affect the polarization-repolarization phase of the action potential, its excitability or refractoriness, or impulse conduction or membrane responsiveness within cardiac fibers. Anti-arrhythmia agents are often classed into four main groups according to their mechanism of action: sodium channel blockade, beta-adrenergic blockade, repolarization prolongation, or calcium channel blockade. (See all compounds classified as Anti-Arrhythmia Agents.)


