
Synopsis
Synopsis
0
KDMF
0
VMF
DRUG PRODUCT COMPOSITIONS
Regulatory FDF Prices
NA
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
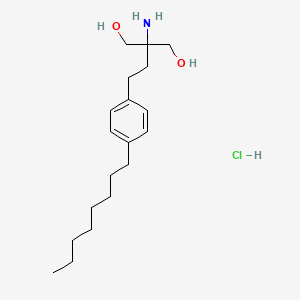

1. 2-amino-2-(2-(4-octylphenyl)ethyl)-1,3-propanediol Hydrochloride
2. Fingolimod
3. Fty 720
4. Fty-720
5. Fty720
6. Gilenia
7. Gilenya
1. 162359-56-0
2. Fty720
3. Fingolimod Hcl
4. Gilenia
5. Gilenya
6. Fty 720
7. Fty-720
8. Fingolimod (hydrochloride)
9. Fingolimod (fty720) Hcl
10. 2-amino-2-(4-octylphenethyl)propane-1,3-diol Hydrochloride
11. Fingolimod, Hcl
12. Fty720 Hydrochloride
13. Fingolimod Hydrochloride [usan]
14. 2-amino-2-(2-(4-octylphenyl)ethyl)-1,3-propanediol Hydrochloride
15. Fty720 Free Base
16. Chebi:63112
17. Fingolimod Hydrochlorid
18. 2-amino-2-[2-(4-octylphenyl)ethyl]propane-1,3-diol;hydrochloride
19. Fingolimod (as Hydrochloride)
20. Fty-720 Hydrochloride
21. 2-amino-2-[2-(4-octylphenyl)ethyl]propane-1,3-diol Hydrochloride
22. G926ec510t
23. Mfcd00939512
24. 2-amino-2-(2-(4-octylphenyl)ethyl)propane-1,3-diol Hydrochloride
25. Gilenya (tn)
26. 1,3-propanediol, 2-amino-2-(2-(4-octylphenyl)ethyl)-, Hydrochloride
27. 1,3-propanediol, 2-amino-2-[2-(4-octylphenyl)ethyl]-, Hydrochloride
28. 2-amino-2-[2-(4-octylphenyl)ethyl]-1,3-propanediol, Hydrochloride
29. Fty-720a
30. Fingolimod (fty720)
31. Imusera
32. Unii-g926ec510t
33. 2-amino-2-[2-(4-octylphenyl)ethyl]-1,3-propanediol Hydrochloride
34. Fty720,fingolimod
35. Fingolimodhydrochloride
36. Fin;limod Hydrochloride
37. Fty720 - Fingolimod
38. Fingolimod Hclfty-720
39. Epitope Id:156573
40. Schembl81362
41. Mls006010179
42. Chembl544665
43. Fingolimod Hydrochloride- Bio-x
44. Dtxsid00167364
45. Ex-a960
46. Tdi-132
47. Bcpp000225
48. Bcp01808
49. 2-amino-2-[2-(4-octyl-phenyl)-ethyl]-propane-1,3-diol Hcl
50. Fingolimod Hydrochloride (jan/usan)
51. Fingolimod Hydrochloride [mi]
52. S5002
53. Fingolimod Hydrochloride [jan]
54. Akos005145784
55. Ac-1929
56. Am84549
57. Bcp9000705
58. Ccg-265016
59. Cs-0114
60. Ks-1172
61. Fingolimod Hydrochloride [usp-rs]
62. Fingolimod Hydrochloride [who-dd]
63. Bf164458
64. Hy-12005
65. Smr004701287
66. Sy057854
67. Db-014816
68. A8548
69. F1018
70. Ft-0643569
71. Sw219384-1
72. Ec-000.2314
73. Fingolimod Hydrochloride [orange Book]
74. A25158
75. D04187
76. Fingolimod Hydrochloride [ep Monograph]
77. Fingolimod Hydrochloride [usp Monograph]
78. 359f560
79. Sr-01000942237
80. Q-101363
81. Sr-01000942237-2
82. Q27132395
83. 2-(4-octylphenethyl)-2-aminopropane-1,3-diol Hydrochloride
84. 2-amino-2-[2-(4-octylphenyl) Ethyl]-1,3-propanediol Hydrochloride
85. 2-amino-2-[2-(4-octylphenyl)-ethyl]-1,3-propanediol Hydrochloride
86. [1-hydroxy-2-(hydroxymethyl)-4-(4-octylphenyl)butan-2-yl]azanium;chloride
87. 2-amino-2-[2-(4-n-octylphenyl)ethyl]propane-1,3-diol Hydrochloride
88. 2-amino-2-[2-(4-octyl-phenyl)-ethyl]-propane -1,3-diol Hydrochloride
89. 2-amino-2-[2-(4-octyl-phenyl)-ethyl]-propane-1,3-diol Hydrochloride
90. 1,3-propanediol, 2-amino-2-[2-(4-octylphenyl)ethyl]-, Hydrochloride (1:1)
| Molecular Weight | 343.9 g/mol |
|---|---|
| Molecular Formula | C19H34ClNO2 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 12 |
| Exact Mass | 343.2278070 g/mol |
| Monoisotopic Mass | 343.2278070 g/mol |
| Topological Polar Surface Area | 66.5 Ų |
| Heavy Atom Count | 23 |
| Formal Charge | 0 |
| Complexity | 258 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 2 | |
|---|---|
| Drug Name | Gilenya |
| PubMed Health | Fingolimod (By mouth) |
| Drug Classes | Immune Modulator |
| Drug Label | Fingolimod is a sphingosine 1-phosphate receptor modulator.Chemically, fingolimod is 2-amino-2-[2-(4-octylphenyl)ethyl]propan-1,3-diol hydrochloride. Its structure is shown below: Fingolimod hydrochloride is a white to practically white powder that i... |
| Active Ingredient | Fingolimod |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 0.5mg |
| Market Status | Prescription |
| Company | Novartis |
| 2 of 2 | |
|---|---|
| Drug Name | Gilenya |
| PubMed Health | Fingolimod (By mouth) |
| Drug Classes | Immune Modulator |
| Drug Label | Fingolimod is a sphingosine 1-phosphate receptor modulator.Chemically, fingolimod is 2-amino-2-[2-(4-octylphenyl)ethyl]propan-1,3-diol hydrochloride. Its structure is shown below: Fingolimod hydrochloride is a white to practically white powder that i... |
| Active Ingredient | Fingolimod |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 0.5mg |
| Market Status | Prescription |
| Company | Novartis |
Gilenya is indicated as single disease modifying therapy in highly active relapsing remitting multiple sclerosis for the following groups of adult patients and paediatric patients aged 10 years and older:
- Patients with highly active disease despite a full and adequate course of treatment with at least one disease modifying therapy (for exceptions and information about washout periods see sections 4. 4 and 5. 1).
or
- Patients with rapidly evolving severe relapsing remitting multiple sclerosis defined by 2 or more disabling relapses in one year, and with 1 or more Gadolinium enhancing lesions on brain MRI or a significant increase in T2 lesion load as compared to a previous recent MRI.
Sphingosine 1 Phosphate Receptor Modulators
Agents that affect the function of G-protein coupled SPHINGOSINE 1-PHOSPHATE RECEPTORS. Their binding to the receptors blocks lymphocyte migration and are often used as IMMUNOSUPPRESSANTS. (See all compounds classified as Sphingosine 1 Phosphate Receptor Modulators.)
Immunosuppressive Agents
Agents that suppress immune function by one of several mechanisms of action. Classical cytotoxic immunosuppressants act by inhibiting DNA synthesis. Others may act through activation of T-CELLS or by inhibiting the activation of HELPER CELLS. While immunosuppression has been brought about in the past primarily to prevent rejection of transplanted organs, new applications involving mediation of the effects of INTERLEUKINS and other CYTOKINES are emerging. (See all compounds classified as Immunosuppressive Agents.)
L04AA27

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Ublituximab is a antibody drug candidate, which is currently being evaluated in Phase II/ Phase III clinical studies for the treatment of Multiple Sclerosis, Relapsing-Remitting.
Lead Product(s): Ublituximab,Fingolimod Hydrochloride
Therapeutic Area: Neurology Brand Name: Undisclosed
Study Phase: Phase II/ Phase IIIProduct Type: Antibody, Unconjugated
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable October 23, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Ublituximab,Fingolimod Hydrochloride
Therapeutic Area : Neurology
Highest Development Status : Phase II/ Phase III
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Ublituximab Effects in Pediatric Multiple Sclerosis Participants
Details : Ublituximab is a antibody drug candidate, which is currently being evaluated in Phase II/ Phase III clinical studies for the treatment of Multiple Sclerosis, Relapsing-Remitting.
Product Name : Undisclosed
Product Type : Antibody, Unconjugated
Upfront Cash : Inapplicable
October 23, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Tascenso (fingolimod) is a sphingosine 1-phosphate receptor modulator indicated for the treatment of relapsing forms of MS, to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in patients 10 years of age and older.
Lead Product(s): Fingolimod Hydrochloride,Inapplicable
Therapeutic Area: Neurology Brand Name: Tascenso
Study Phase: Approved FDFProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable February 13, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Fingolimod Hydrochloride,Inapplicable
Therapeutic Area : Neurology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
New Drug, Tascenso ODT® (fingolimod), Launched to Meet the Needs of Multiple Sclerosis Patients i...
Details : Tascenso (fingolimod) is a sphingosine 1-phosphate receptor modulator indicated for the treatment of relapsing forms of MS, to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in patients 10 yea...
Product Name : Tascenso
Product Type : Miscellaneous
Upfront Cash : Inapplicable
February 13, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Tascenso (fingolimod) is a sphingosine 1-phosphate receptor modulator indicated for the treatment of relapsing forms of MS, to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in patients 10 years of age and older.
Lead Product(s): Fingolimod Hydrochloride,Inapplicable
Therapeutic Area: Neurology Brand Name: Tascenso
Study Phase: Approved FDFProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable January 17, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Fingolimod Hydrochloride,Inapplicable
Therapeutic Area : Neurology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : Tascenso (fingolimod) is a sphingosine 1-phosphate receptor modulator indicated for the treatment of relapsing forms of MS, to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in patients 10 yea...
Product Name : Tascenso
Product Type : Miscellaneous
Upfront Cash : Inapplicable
January 17, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Fingolimod, an orally available immunomodulatory drug, is a sphingosine 1-phosphate receptor modulator used to treat patients with the relapsing-remitting form of multiple sclerosis (MS).
Lead Product(s): Fingolimod Hydrochloride,Inapplicable
Therapeutic Area: Neurology Brand Name: Gilenya-Generic
Study Phase: Approved FDFProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable October 31, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Fingolimod Hydrochloride,Inapplicable
Therapeutic Area : Neurology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Glenmark Pharmaceuticals launches Fingolimod Capsules, 0.5 mg in the US
Details : Fingolimod, an orally available immunomodulatory drug, is a sphingosine 1-phosphate receptor modulator used to treat patients with the relapsing-remitting form of multiple sclerosis (MS).
Product Name : Gilenya-Generic
Product Type : Miscellaneous
Upfront Cash : Inapplicable
October 31, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
GILENYA (fingolimod) is a prescription medicine used to treat relapsing forms of multiple sclerosis (MS), to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in adults and children 10 years of age and older.
Lead Product(s): Fingolimod Hydrochloride,Inapplicable
Therapeutic Area: Neurology Brand Name: Gilenya
Study Phase: Approved FDFProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable September 21, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Fingolimod Hydrochloride,Inapplicable
Therapeutic Area : Neurology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Novartis Plans To Petition The U.S. Supreme Court To Uphold Validity Of The Gilenya® (Fingolimod)...
Details : GILENYA (fingolimod) is a prescription medicine used to treat relapsing forms of multiple sclerosis (MS), to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in adults and children 10 years of a...
Product Name : Gilenya
Product Type : Miscellaneous
Upfront Cash : Inapplicable
September 21, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Mean reduction in ADSI score from baseline was -4.05 (-60.62% mean reduction) for FMX114 (tofacitinib) treated lesions compared to -3.48 (-51.32% mean reduction) for vehicle treated lesions at week 4 (p=0.228, OC, ITT).
Lead Product(s): Tofacitinib Citrate,Fingolimod Hydrochloride
Therapeutic Area: Dermatology Brand Name: Undisclosed
Study Phase: Phase I/ Phase IIProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable August 10, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Tofacitinib Citrate,Fingolimod Hydrochloride
Therapeutic Area : Dermatology
Highest Development Status : Phase I/ Phase II
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : Mean reduction in ADSI score from baseline was -4.05 (-60.62% mean reduction) for FMX114 (tofacitinib) treated lesions compared to -3.48 (-51.32% mean reduction) for vehicle treated lesions at week 4 (p=0.228, OC, ITT).
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
August 10, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Gilenya® (fingolimod), an oral, once-daily at 0.5mg and 0.25mg versus once-daily subcutaneous injections of glatiramer acetate 20mg in reducing disease activity over 12 months in patients with relapsing remitting multiple sclerosis (RRMS).
Lead Product(s): Fingolimod Hydrochloride,Inapplicable
Therapeutic Area: Neurology Brand Name: Gilenya
Study Phase: Approved FDFProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable June 21, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Fingolimod Hydrochloride,Inapplicable
Therapeutic Area : Neurology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : Gilenya® (fingolimod), an oral, once-daily at 0.5mg and 0.25mg versus once-daily subcutaneous injections of glatiramer acetate 20mg in reducing disease activity over 12 months in patients with relapsing remitting multiple sclerosis (RRMS).
Product Name : Gilenya
Product Type : Miscellaneous
Upfront Cash : Inapplicable
June 21, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
FMX114 (tofacitinib), is VYNE’s proprietary investigational combination gel formulation of tofacitinib and fingolimod, which has been designed to address both the source and cause of inflammation in AD.
Lead Product(s): Tofacitinib Citrate,Fingolimod Hydrochloride
Therapeutic Area: Dermatology Brand Name: Undisclosed
Study Phase: Phase I/ Phase IIProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable June 17, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Tofacitinib Citrate,Fingolimod Hydrochloride
Therapeutic Area : Dermatology
Highest Development Status : Phase I/ Phase II
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : FMX114 (tofacitinib), is VYNE’s proprietary investigational combination gel formulation of tofacitinib and fingolimod, which has been designed to address both the source and cause of inflammation in AD.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
June 17, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
FMX114, an investigational combination gel formulation of tofacitinib and fingolimod, demonstrated a statistically significant reduction in both absolute and percent change in ADSI score compared to vehicle at week 2.
Lead Product(s): Tofacitinib Citrate,Fingolimod Hydrochloride
Therapeutic Area: Dermatology Brand Name: Undisclosed
Study Phase: Phase I/ Phase IIProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable April 07, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Tofacitinib Citrate,Fingolimod Hydrochloride
Therapeutic Area : Dermatology
Highest Development Status : Phase I/ Phase II
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : FMX114, an investigational combination gel formulation of tofacitinib and fingolimod, demonstrated a statistically significant reduction in both absolute and percent change in ADSI score compared to vehicle at week 2.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
April 07, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
HEC Pharma filed the ANDA to get generic version of Novartis' Gilenya® (fingolimod), in response to that Novartis filed litigation against ANDA filers for US Patent No. 9,187,405 and extend the validity of Gilenya till 2027.
Lead Product(s): Fingolimod Hydrochloride,Inapplicable
Therapeutic Area: Neurology Brand Name: Gilenya
Study Phase: Approved FDFProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable January 04, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Fingolimod Hydrochloride,Inapplicable
Therapeutic Area : Neurology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : HEC Pharma filed the ANDA to get generic version of Novartis' Gilenya® (fingolimod), in response to that Novartis filed litigation against ANDA filers for US Patent No. 9,187,405 and extend the validity of Gilenya till 2027.
Product Name : Gilenya
Product Type : Miscellaneous
Upfront Cash : Inapplicable
January 04, 2022

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
18
PharmaCompass offers a list of Fingolimod Hydrochloride API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Fingolimod Hydrochloride manufacturer or Fingolimod Hydrochloride supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Fingolimod Hydrochloride manufacturer or Fingolimod Hydrochloride supplier.
PharmaCompass also assists you with knowing the Fingolimod Hydrochloride API Price utilized in the formulation of products. Fingolimod Hydrochloride API Price is not always fixed or binding as the Fingolimod Hydrochloride Price is obtained through a variety of data sources. The Fingolimod Hydrochloride Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Fingolimod manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Fingolimod, including repackagers and relabelers. The FDA regulates Fingolimod manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Fingolimod API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Fingolimod manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Fingolimod supplier is an individual or a company that provides Fingolimod active pharmaceutical ingredient (API) or Fingolimod finished formulations upon request. The Fingolimod suppliers may include Fingolimod API manufacturers, exporters, distributors and traders.
click here to find a list of Fingolimod suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Fingolimod DMF (Drug Master File) is a document detailing the whole manufacturing process of Fingolimod active pharmaceutical ingredient (API) in detail. Different forms of Fingolimod DMFs exist exist since differing nations have different regulations, such as Fingolimod USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Fingolimod DMF submitted to regulatory agencies in the US is known as a USDMF. Fingolimod USDMF includes data on Fingolimod's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Fingolimod USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Fingolimod suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Fingolimod Drug Master File in Japan (Fingolimod JDMF) empowers Fingolimod API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Fingolimod JDMF during the approval evaluation for pharmaceutical products. At the time of Fingolimod JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Fingolimod suppliers with JDMF on PharmaCompass.
A Fingolimod CEP of the European Pharmacopoeia monograph is often referred to as a Fingolimod Certificate of Suitability (COS). The purpose of a Fingolimod CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Fingolimod EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Fingolimod to their clients by showing that a Fingolimod CEP has been issued for it. The manufacturer submits a Fingolimod CEP (COS) as part of the market authorization procedure, and it takes on the role of a Fingolimod CEP holder for the record. Additionally, the data presented in the Fingolimod CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Fingolimod DMF.
A Fingolimod CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Fingolimod CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Fingolimod suppliers with CEP (COS) on PharmaCompass.
A Fingolimod written confirmation (Fingolimod WC) is an official document issued by a regulatory agency to a Fingolimod manufacturer, verifying that the manufacturing facility of a Fingolimod active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Fingolimod APIs or Fingolimod finished pharmaceutical products to another nation, regulatory agencies frequently require a Fingolimod WC (written confirmation) as part of the regulatory process.
click here to find a list of Fingolimod suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Fingolimod as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Fingolimod API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Fingolimod as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Fingolimod and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Fingolimod NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Fingolimod suppliers with NDC on PharmaCompass.
Fingolimod Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Fingolimod GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Fingolimod GMP manufacturer or Fingolimod GMP API supplier for your needs.
A Fingolimod CoA (Certificate of Analysis) is a formal document that attests to Fingolimod's compliance with Fingolimod specifications and serves as a tool for batch-level quality control.
Fingolimod CoA mostly includes findings from lab analyses of a specific batch. For each Fingolimod CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Fingolimod may be tested according to a variety of international standards, such as European Pharmacopoeia (Fingolimod EP), Fingolimod JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Fingolimod USP).

