



API Suppliers

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
Listed Suppliers

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
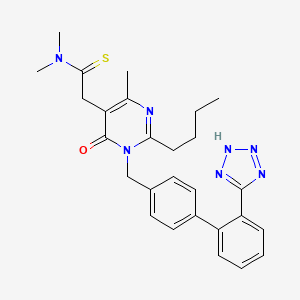

1. Br-a657
1. 247257-48-3
2. Kanarb
3. Fimasartan [inn]
4. 2-(1-((2'-(2h-tetrazol-5-yl)-[1,1'-biphenyl]-4-yl)methyl)-2-butyl-4-methyl-6-oxo-1,6-dihydropyrimidin-5-yl)-n,n-dimethylethanethioamide
5. Chembl1951143
6. Fimasartan (inn)
7. P58222188p
8. 2-[2-butyl-4-methyl-6-oxo-1-[[4-[2-(2h-tetrazol-5-yl)phenyl]phenyl]methyl]pyrimidin-5-yl]-n,n-dimethylethanethioamide
9. 2-(2-butyl-4-methyl-6-oxo-1-{[2'-(1h-tetrazol-5-yl)-4-biphenylyl]methyl}-1,6-dihydro-5-pyrimidinyl)-n,n-dimethylethanethioamide
10. Br-a657
11. Br-a-657.k
12. Unii-p58222188p
13. 5-pyrimidineethanethioamide, 2-butyl-1,6-dihydro-n,n,4-trimethyl-6-oxo-1-((2'-(2h-tetrazol-5-yl)(1,1'-biphenyl)-4-yl)methyl)-
14. 5-pyrimidineethanethioamide, 2-butyl-1,6-dihydro-n,n,4-trimethyl-6-oxo-1-[[2'-(2h-tetrazol-5-yl)[1,1'-biphenyl]-4-yl]methyl]-
15. Fimasartan [who-dd]
16. Schembl2229436
17. Schembl20126833
18. Dtxsid80179460
19. Chebi:136044
20. Bcp11616
21. Ex-a3997
22. Hy-b0780
23. Zinc3842872
24. Bdbm50364573
25. Mfcd13194795
26. S4975
27. Zb1816
28. Akos016011331
29. Akos037643761
30. Ccg-269705
31. Cs-3509
32. Db09279
33. Ncgc00390600-01
34. Ac-30631
35. As-35179
36. D10556
37. 257f483
38. A919293
39. L019170
40. Q8563179
41. 2-((2-butyl-4-methyl-6-oxo-1-((2'-(1h-tetrazol-5-yl)biphenyl-4-yl)methyl)-1,6-dihydropyrimidin-5-yl))-n,n-dimethylthioacetamide
42. 2-(1-((2'-(1h-tetrazol-5-yl)biphenyl-4-yl)methyl)-2-butyl-4-methyl-6-oxo-1,6-dihydropyrimidin-5-yl)-n,n-dimethylethanethioamide
43. 2-(1-((2-(2h-tetrazol-5-yl)-[1,1-biphenyl]-4-yl)methyl)-2-butyl-4-methyl-6-oxo-1,6-dihydropyrimidin-5-yl)-n,n-dimethylethanethioamide
44. 2-[2-butyl-4-methyl-6-oxo-1-[[4-[2-(2h-tetrazol-5-yl)phenyl]phenyl]met Hyl]pyrimidin-5-yl]-n,n-dimethylethanethioamide
45. 2-butyl-5-dimethylaminothiocarbonylmethyl-6-methyl-3-[[2'-(1h-tetrazol-5-yl)biphenyl-4-yl]methyl]pyrimidin-4(3h)-one
| Molecular Weight | 501.6 g/mol |
|---|---|
| Molecular Formula | C27H31N7OS |
| XLogP3 | 3.5 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 9 |
| Exact Mass | 501.23107981 g/mol |
| Monoisotopic Mass | 501.23107981 g/mol |
| Topological Polar Surface Area | 123 Ų |
| Heavy Atom Count | 36 |
| Formal Charge | 0 |
| Complexity | 849 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Used for the treatment of hypertension and heart failure.
Fimasartan is a selective angiotensin receptor 1 (AR1) inhibitor. It acts to lower blood pressure by inhibiting vasoconstriction
C - Cardiovascular system
C09 - Agents acting on the renin-angiotensin system
C09C - Angiotensin ii receptor blockers (arbs), plain
C09CA - Angiotensin ii receptor blockers (arbs), plain
C09CA10 - Fimasartan
Absorption
Tmax is 0.5-1.3 h.
Route of Elimination
Most is eliminated unchangd in bile with less than 3% in the urine.
The half life of elimination is 7-10 h.
Angiotensin II activates AR1 leading to vasoconstriction and increased noradrenaline release which further increases vasoconstriction via action at 1-adrenergic receptors. It also stimulates secretion of aldosterone which acts to increase sodium and water reabsorption in the renal tubules. Fimasartan bind to and antagonizes AR1 preventing vasoconstriction and reducing aldosterone secretion to increase natriuresis leading to a reduction in blood volume. Together these effects produce an anti-hypertensive effect.


