
Synopsis
Synopsis
0
EU WC
0
KDMF
0
VMF
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
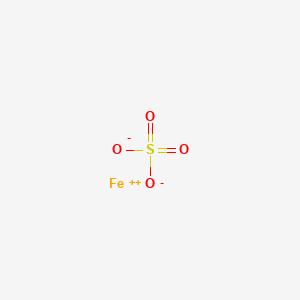

1. Aktiferrin
2. Apo-ferrous Sulfate
3. Biofer
4. Ceferro
5. Conferon
6. Eisendragees-ratiopharm
7. Eisensulfat Stada
8. Feospan
9. Fer-gen-sol
10. Fer-in-sol
11. Feratab
12. Fero-gradumet
13. Ferodan
14. Ferogradumet
15. Ferro-gradumet
16. Ferrogamma
17. Ferrograd
18. Ferroinfant
19. Ferrous Sulfate, Heptahydrate
20. Ferrous Sulphate
21. Hmatopan
22. Haemoprotect
23. Hemobion
24. Hemofer
25. Iron(ii) Sulfate Heptahydrate
26. Kendural
27. Mol-iron
28. Plastufer
29. Slow-fe
30. Vitaferro Kapseln
1. Ferrous Salt
2. Iron(ii) Sulfate
3. 7720-78-7
4. Iron Sulfate
5. Iron(2+) Sulfate
6. Iron Sulphate
7. Ferrous Sulfate Anhydrous
8. Iron Sulfate (1:1)
9. Iron(2+) Sulphate
10. Feso4
11. Iron(ii) Sulfate (1:1)
12. Iron Sulfate (feso4)
13. Ferrous Sulfate (1:1)
14. Sulfuric Acid, Iron(2+) Salt (1:1)
15. Sulfuric Acid, Iron(2+) Salt
16. 2idp3x9oud
17. 16547-58-3
18. Iron(2+) Sulfate (anhydrous)
19. Iron Vitriol; Iron(2+) Sulfate
20. Combiron
21. Odophos
22. Kesuka
23. Sal Chalybis
24. Green Salts
25. Quickfloc (salt)
26. Slow Fe
27. Ferrosulfat [german]
28. Ferrosulfat
29. Ccris 6796
30. Hsdb 465
31. Sfe 171
32. Ferrous Sulfate Solution
33. Einecs 231-753-5
34. Unii-2idp3x9oud
35. Nsc 57631
36. Iron(ii) Sulfate Solution
37. Nsc 146177
38. Ai3-51903
39. Ferrous Sulphate Anhydrous
40. Iron(ii)sulphate
41. Fe(ii) Sulphate
42. Iron(ii) Sulphate
43. Einecs 240-616-9
44. Iron(2+);sulfate
45. Iron (as Sulphate)
46. Iron (ii) Sulphate
47. Ferrous Sulfate,dried
48. Errous Hydrogen Sulfide
49. Sulfuric Acid, Iron(2+) Salt (1:?)
50. Iron Sulphate (feso4)
51. Ferrous Sulfate, 98%
52. Ferrous Sulfate (anh.)
53. Fe(ii)so4
54. Dsstox_cid_9688
55. Ferrous Sulfate, Anhydrous
56. Ferrous Sulphate (1:1)
57. Ec 231-753-5
58. Ferrous Sulphate, Anhydrous
59. Ferrous Sulfate (anhydrous)
60. Dsstox_rid_78808
61. Iron(2+) Sulfate (anh.)
62. Dsstox_gsid_29688
63. Ferrous Sulfate [mi]
64. Iron(ii) Sulfate (feso4)
65. Ferrous Sulfate [iso]
66. Ferrous Sulfate [hsdb]
67. Dtxsid0029688
68. Chebi:75832
69. Ferrous Sulfate [who-dd]
70. Ferrous Sulphate Exsiccated (dried)
71. Iron(ii) Sulphate (1:1)
72. Tox21_202580
73. Ferrous Sulfate,dried [vandf]
74. Db13257
75. Ncgc00260129-01
76. Cas-7720-78-7
77. Ft-0626420
78. Q214863
79. 8063-79-4
| Molecular Weight | 151.91 g/mol |
|---|---|
| Molecular Formula | FeO4S |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 0 |
| Exact Mass | 151.886665 g/mol |
| Monoisotopic Mass | 151.886665 g/mol |
| Topological Polar Surface Area | 88.6 Ų |
| Heavy Atom Count | 6 |
| Formal Charge | 0 |
| Complexity | 62.2 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Initial response to iron therapy ...will confirm or negate diagnosis of iron-deficiency anemia. Ferrous sulfate, 150-300 mg thrice daily, is given for 3 wk. Beginning about a wk after starting therapy, circulating hemoglobin should rise about 0.1-0.3 g % daily; less severe anemia, less daily rise.
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 1290
Supplementation with 30-60 mg of iron daily (ie, 150-300 mg of ferrous sulfate) has been advocated for pregnant women ...and 0.3-0.6 mL of ferrous sulfate pediatric "drops" daily for low-birth-weight infants from 1 month until 1 yr of age. Physician must use his judgement in this regard... .
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 1290
Hematinic.
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001., p. 717
MEDICATION (VET): In iron deficiency. Astringent.
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001., p. 717
For more Therapeutic Uses (Complete) data for FERROUS SULFATE (11 total), please visit the HSDB record page.
... A double-lumen perfusion tube was positioned orogastrically into a 40-cm segment of the proximal small intestine in six healthy volunteers (25 +/- 5 yr). The segment was perfused with saline and subsequently with saline containing 80 mg iron as ferrous sulfate at a rate of 10 mL/min. Intestinal fluid samples were collected at 15-min intervals. Thiobarbituric acid reactive substances concentrations as an indicator of lipid peroxidation increased significantly from 0.07 uM (range, 0-0.33 uM) during saline perfusion to 3.35 uM (range, 1.19-7.27 uM) during iron perfusion (P<0.05). Nonprotein antioxidant capacity increased significantly from 474 uM (range, 162-748 uM) to 1,314 uM (range, 674-1,542 uM) (P<0.05). These data show that a single dosage of ferrous sulfate induces oxidative damage and the subsequent release of an antioxidant in the small intestine in vivo in healthy volunteers.
Troost FJ et al; Am J Physiol Gastrointest Liver Physiol 285 (2): G354-9 (2003)
Adverse effects of ferrous sulfate are those of iron compounds in general, but they are rarely severe when drug is taken in therapeutic doses; however, relatively small overdoses can cause serious intoxication in infants and children.
Osol, A. (ed.). Remington's Pharmaceutical Sciences. 16th ed. Easton, Pennsylvania: Mack Publishing Co., 1980., p. 780
VET: Administer with or after feeding to help avoid gastritis.
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 221
Adverse effects are generally dose dependent; in 10% of patients they are severe enough to be intolerable. Gastrointestinal disturbances ... are most common. Ferrous sulfate is absorbed best when taken between meals, but gastrointestinal symptoms may be minimized by reducing the dose and/or giving it in divided amounts with meals or shortly thereafter. In some patients, admin of one-half the total daily dose at bedtime improves tolerance. ... Large doses of ferrous sulfate may aggravate existing gastrointestinal diseases ... . Acute severe iron poisoning is uncommon in adults but does occur in children who ingest formulations intended for adults. In young children, as little as 400 mg of elemental iron is potentially fatal.
American Medical Association, AMA Department of Drugs. AMA Drug Evaluations. 5th ed. Chicago: American Medical Association, 1983., p. 797
For more Drug Warnings (Complete) data for FERROUS SULFATE (9 total), please visit the HSDB record page.
Ferrous sulfate is used for the prevention and treatment of iron deficiency anemia in adults and children.
Ferrous sulfate replenishes iron, an essential component in hemoglobin, myoglobin, and various enzymes. It replaces the iron that is usually found in hemoglobin and myoglobin. Iron participates in oxygen transport and storage, electron transport and energy metabolism, antioxidant and beneficial pro-oxidant functions, oxygen sensing, tissue proliferation and growth, as well as DNA replication and repair.
B - Blood and blood forming organs
B03 - Antianemic preparations
B03A - Iron preparations
B03AA - Iron bivalent, oral preparations
B03AA07 - Ferrous sulfate
B - Blood and blood forming organs
B03 - Antianemic preparations
B03A - Iron preparations
B03AD - Iron in combination with folic acid
B03AD03 - Ferrous sulfate
Absorption
Approximately 5 10% of dietary iron is absorbed, and this absorption rate increases to up to 30% in iron deficiency states. Oral iron supplements are absorbed up to 60% via active and passive transport processes. Gastrointestinal absorption of iron occurs via strict regulation by the enterocyte and duodenal cytochrome and ferric reductase enzymes. The hormone hepcidin heavily regulates iron absorption and distribution throughout the body. The median time to maximum serum concentration (Tmax) is generally 4 hours after administration. Between 2-8 hours post administration, average serum iron concentrations fluctuate by 20%, according to one study. Bioavailability of iron depends on whether it is administered in a film coated tablet or enteric coated tablet. One pharmacokinetic study in healthy volunteers revealed a 30% bioavailability for enteric coated tablets. The AUC of enteric coated tablets varied between a lower limit of -46.93 to 5.25 molxh/l. Cmax is higher for film coated tablets, ranging from 3.4 to 22.1 mol/h/l. It is advisable to take ferrous sulfate with ascorbic acid, as this practice may increase absorption. Avoid antacids, tea, coffee,tea, dairy products, eggs, and whole-grain bread for at least an hour after taking ferrous sulfate. Calcium can decrease iron absorption by 33% if taken concomitantly.
Route of Elimination
Oral iron is recycled, with some loss in the urine, sweat, and desquamation. Some iron can be lost during menstrual bleeding This loss is balanced by changes in intestinal absorption. The enzyme hepcidin promotes the excretion of iron via the sloughing of enterocytes with ferritin stores into the feces.
Volume of Distribution
About 60% of iron is distributed the erythrocytes. The remainder of the iron is found in muscle tissues (as a part of myoglobin), and in a variety of different enzymes, as well as in storage form. Most stored iron is in the form of ferritin, which can be found in the liver, bone marrow, spleen and, and muscle. Iron crosses the placenta and is also found in breast milk.
... The bioavailability studies were carried out using four groups of 30 female mice each. In two groups, we studied the absorption of ferrous ascorbate and ferrous sulfate, both in water as reference standards, which show absorptions of 13.1+/-4.9% and 13.2+/-4.3%, respectively. With the third group, we studied the absorption of ferrous sulfate in milk; its value, 7.9+/-3.2%, is significantly lower than that of the remaining groups, with a p < 0.01. The studies with SFE-171 in milk, were performed on the fourth group, with a result of 11.6+/-4.5%, demonstrating that its absorption does not differ significantly from that of the reference standards. The absorption mechanism was determined by means of in vivo self-displacement studies of the ferrous ion and the SFE-171, taking ferrous sulfate as the reference compound. For this study, 210 female mice were used, and no significant difference between the absorption mechanism of both products could be observed.
PMID:9630425 Boccio JR et al; Biol Trace Elem Res 62 (1-2): 65-73 (1998)
We investigated the iron bioavailability of microencapsulated ferrous sulfate (SFE-171) in a diet based on powdered milk by using the prophylactic method in rats.The SFE-171 was added into fluid milk and industrially processed into powdered milk, which was then mixed in our laboratory with a normalized diet (17.2 +/- 2.1 mg Fe/kg). A reference standard diet using ferrous sulfate as iron-fortifying source (19.8 p+/- 2.9 mg Fe/kg) and a control diet without added iron (4.6 +/- 0.8 mg Fe/kg) were prepared in the laboratory in a similar way. These diets were administered to different groups of weaning rats for 28 d as the only solid nourishment. The iron bioavailability of the different sources was calculated as the relation between the mass of iron incorporated into hemoglobin during the treatment and the total iron intake per animal. The iron bioavailability values of SFE-171 and ferrous sulfate in the fortified diets were 41.6 +/- 6.6% and 42.6 +/- 4.2%, respectively; these results were significantly higher (P < 0.01) than the iron bioavailability of the control diet (28.8 +/- 8.1%).
Lysionek AE et al; Nutr 18 (3): 279-281 (2002)
A prospective analytical study was conducted to determine the relationship between nonprotein-bound iron and serum iron concentrations following gastric instillation of ferrous sulfate. Four female pigs (2022 kg) with indwelling central venous lines and gastrostomy tubes were studied. A 5% solution of ferrous sulfate (20 mg elemental iron/kg bwt) was administered through the gastrostomy tube over 1 to 2 min. Six hourly blood samples were collected, and serum samples were subjected to ultrafiltration with the filtrate representing nonprotein-bound iron. Iron concentrations were determined by atomic absorption spectrophotometry. Baseline (mean | SD) iron concentrations were 73 | 25 mug/dL as the serum total and 21 | 4 mug/dL as nonprotein-bound iron. The serum iron and nonprotein-bound iron concentrations achieved a peak of 191 | 66 and 23 | 10, respectively, at 2 h and declined to near baseline values at 6 h. The mean ratio of filtrate to serum iron concentration was 0.
PMID:8825744 Chyka PA et al; Vet Hum Toxicol 38 (1): 24-26 (1996)
Gastrointestinal absorption of iron is adequate and essentially equal from...ferrous...sulfate, fumarate, gluconate, succinate, glutamate, and lactate.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1315
The metabolism of iron is complex. Normally, iron exists in the ferrous (Fe2+) or ferric (Fe3+) state, but since Fe2+ is oxidized to Fe3+, which hydrolyzes to insoluble iron(III)hydroxides in neutral aqueous solutions, iron binds to plasma proteins and is either transported or stored throughout the body. There are three proteins that serve to regulate the storage and transport of ingested iron. The first protein , transferrin, transports iron in both the plasma and extracellular fluid. Ceruloplasmin in the plasma and hephaestin on the enterocyte participate in the oxidation and binding of iron to transferrin. The main role of transferrin is the chelation of iron to prevent the production of reactive oxygen species, while facilitating its transport into cells. The transferrin receptor, located on many cells that require iron, binds the transferrin complex and internalizes this complex. Ferritin is a protein that stores iron, making it readily available for body requirements.
The half-life of orally administered iron is not readily available in the literature, with total effects lasting 2-4 months (congruent with the red blood cell life span) with an onset of action of 4 days and peak activity at 7-10 days.
Iron is required to maintain optimal health, particularly for helping to form red blood cells (RBC) that carry oxygen around the body. A deficiency in iron indicates that the body cannot produce enough normal red blood cells. Iron deficiency anemia occurs when body stores of iron decrease to very low levels, and the stored iron is insufficient to support normal red blood cell (RBC) production. Insufficient dietary iron, impaired iron absorption, bleeding, pregnancy, or loss of iron through the urine can lead to iron deficiency. Symptoms of iron deficiency anemia include fatigue, breathlessness, palpitations, dizziness, and headache. Taking iron in supplement form, such as ferrous sulfate, allows for more rapid increases in iron levels when dietary supply and stores are not sufficient. Iron is transported by the divalent metal transporter 1 (DMT1) across the endolysosomal membrane to enter the macrophage. It can then can be incorporated into ferritin and be stored in the macrophage or carried of the macrophage by ferroportin. This exported iron is oxidized by the enzyme to ceruloplasmin to Fe3+, followed by sequestration by transferrin for transport in the serum to various sites, including the bone marrow for hemoglobin synthesis or into the liver. Iron combines with porphyrin and globin chains to form hemoglobin, which is critical for oxygen delivery from the lungs to other tissues.
Coagulopathy is a hallmark of severe ferrous sulfate poisoning in humans and lab animals. At iron concn comparable to those of previous animal investigations, the coagulopathy, in other words, the dose-related prolongation of the prothrombin, thrombin, and partial thromboplastin time, was reproduced in human plasma in vitro. Studies of the mechanism by which iron prevents a normal plasma coagulation revealed that the proenzymes of the coagulation cascade and fibrinogen were not damaged by iron. Fibrinogen coagulability and fibrin monomer aggregation were unaffected by very high iron concn. Instead, thrombin was markedly inhibited by iron in its clotting effect on fibrinogen and, specifically, in its fibrinopeptide A-generating capacity, the inhibitory effect being reversible upon iron removal by ethylenediaminetetraacetic acid chelation and gel filtration. Thrombin generation in the presence of iron was reduced as well, indicating an inhibition of one or several other enzymes of the intrinsic coagulation cascade. Because the amidolytic activity of human thrombin as well as factor Xa, kallikrein, and bovine trypsin was also reversibly suppressed by ferrous sulfate, it is considered likely that coagulopathy occurring in iron poisoning is the consequence of a general, physiologically important phenomenon: the susceptibility of serine proteases to nontransferrin-bound iron(3+)
PMID:6421970 Rosenmund A et al; J Lab Clin Med 103 (4): 524-33 (1984)
The mechanism of acute iron cardiotoxicity was investigated in isometrically contracting left atrial strips and right ventricular papillary muscles isolated from rabbit hearts. A 90 min exposure to iron (1.8 mM; as ferrous sulfate) reduced the peak-developed tension and the maximal rate of tension development.
PMID:6695379 Artman M et al; Toxicol Appl Pharmacol 72 (2): 324-32 (1984)
... Iron is stored in the liver in the oxidized or ferric state & is tightly bound to protein as ferric ferritin. Xanthine oxidase appears to be involved in the conversion of ferric ferritin to ferrous ferritin. The reduced form of iron is less tightly bound to ferritin and thus is more easily released for utilization. Therefore, a possible inverse relationship between hepatic xanthine oxidase activity & hepatic iron storage exists. Theoretically, the xanthine oxidase inhibitor allopurinol should decrease the activity of xanthine oxidase & increase hepatic iron storage.
Evaluations of Drug Interactions. 2nd ed. and supplements. Washington, DC: American Pharmaceutical Assn., 1976, 1978., p. 16/7
 Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.
Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.
 Tenatra connects Indian manufacturers with global buyers through active partners in Germany, Switzerland, Belgium, Spain & Turkey.
Tenatra connects Indian manufacturers with global buyers through active partners in Germany, Switzerland, Belgium, Spain & Turkey.

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 31742
Submission : 2017-05-02
Status : Active
Type : II


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 34465
Submission : 2020-01-31
Status : Active
Type : II


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 29932
Submission : 2015-03-25
Status : Active
Type : II


Certificate Number : R1-CEP 2007-368 - Rev 04
Issue Date : 2022-01-28
Type : Chemical
Substance Number : 2340
Status : Valid

Registration Number : 221MF10113
Registrant's Address : Hauptstrasse 2 D-31860 Emmerthal Germany
Initial Date of Registration : 2009-06-10
Latest Date of Registration :



Certificate Number : R1-CEP 2007-369 - Rev 03
Issue Date : 2022-02-01
Type : Chemical
Substance Number : 83
Status : Valid

Registration Number : 218MF10655
Registrant's Address : Hauptstrasse 2 D-31860 Emmerthal Germany
Initial Date of Registration : 2006-07-24
Latest Date of Registration :

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 31742
Submission : 2017-05-02
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 34465
Submission : 2020-01-31
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 29932
Submission : 2015-03-25
Status : Active
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : R1-CEP 2007-369 - Rev 03
Status : Valid
Issue Date : 2022-02-01
Type : Chemical
Substance Number : 83

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Ferrous Sulfate, Dried, Powder, Milled Powder, M...
Certificate Number : R1-CEP 2007-368 - Rev 04
Status : Valid
Issue Date : 2022-01-28
Type : Chemical
Substance Number : 2340

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Details:
HydroCurc is a Dietary Supplement drug candidate, which is currently being evaluated in clinical studies for the treatment of Iron Deficiencies.
Lead Product(s): HydroCurc,Ferrous Sulfate
Therapeutic Area: Hematology Brand Name: Undisclosed
Study Phase: UndisclosedProduct Type: Dietary Supplement
Sponsor: Pharmako Biotechnologies Pty Ltd
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable July 22, 2025

Lead Product(s) : HydroCurc,Ferrous Sulfate
Therapeutic Area : Hematology
Highest Development Status : Undisclosed
Partner/Sponsor/Collaborator : Pharmako Biotechnologies Pty Ltd
Deal Size : Inapplicable
Deal Type : Inapplicable
Modulation of Hepcidin with Iron & Curcumin in Recreational Athletes
Details : HydroCurc is a Dietary Supplement drug candidate, which is currently being evaluated in clinical studies for the treatment of Iron Deficiencies.
Product Name : Undisclosed
Product Type : Dietary Supplement
Upfront Cash : Inapplicable
July 22, 2025

Details:
Ferrous Sulfate is a Vitamins/Minerals/Inorganic Salts drug candidate, which is currently being evaluated in clinical studies for the treatment of Iron Deficiencies.
Lead Product(s): Ferrous Sulfate,Inapplicable
Therapeutic Area: Hematology Brand Name: Undisclosed
Study Phase: UndisclosedProduct Type: Vitamins/Minerals/Inorganic Salts
Sponsor: Mitacs | Natural Sciences and Engineering Research Council, Canada
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable April 24, 2025

Lead Product(s) : Ferrous Sulfate,Inapplicable
Therapeutic Area : Hematology
Highest Development Status : Undisclosed
Partner/Sponsor/Collaborator : Mitacs | Natural Sciences and Engineering Research Council, Canada
Deal Size : Inapplicable
Deal Type : Inapplicable
Improving Iron Levels in Female Endurance, Intermittent, and Power/Strength Athletes Aged 16-35
Details : Ferrous Sulfate is a Vitamins/Minerals/Inorganic Salts drug candidate, which is currently being evaluated in clinical studies for the treatment of Iron Deficiencies.
Product Name : Undisclosed
Product Type : Vitamins/Minerals/Inorganic Salts
Upfront Cash : Inapplicable
April 24, 2025

Details:
Ferrous Sulfate is a Vitamins/Minerals/Inorganic Salts drug candidate, which is currently being evaluated in clinical studies for the treatment of Low Birth Weight.
Lead Product(s): Ferrous Sulfate,Inapplicable
Therapeutic Area: Nutrition and Weight Loss Brand Name: Undisclosed
Study Phase: UndisclosedProduct Type: Vitamins/Minerals/Inorganic Salts
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable August 15, 2024

Lead Product(s) : Ferrous Sulfate,Inapplicable
Therapeutic Area : Nutrition and Weight Loss
Highest Development Status : Undisclosed
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Pilot Trial Investigating Every Other Day Dosing of Oral Iron in Premature Infants (IQONic)
Details : Ferrous Sulfate is a Vitamins/Minerals/Inorganic Salts drug candidate, which is currently being evaluated in clinical studies for the treatment of Low Birth Weight.
Product Name : Undisclosed
Product Type : Vitamins/Minerals/Inorganic Salts
Upfront Cash : Inapplicable
August 15, 2024

Details:
Ferrous Sulfate is a Vitamins/Minerals/Inorganic Salts drug candidate, which is currently being evaluated in clinical studies for the treatment of Iron Deficiencies.
Lead Product(s): Ferrous Sulfate,Inapplicable
Therapeutic Area: Hematology Brand Name: Undisclosed
Study Phase: UndisclosedProduct Type: Vitamins/Minerals/Inorganic Salts
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable January 11, 2022

Lead Product(s) : Ferrous Sulfate,Inapplicable
Therapeutic Area : Hematology
Highest Development Status : Undisclosed
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Daily Oral Iron Supplementation for Replenishment of Depleted Iron in Adults
Details : Ferrous Sulfate is a Vitamins/Minerals/Inorganic Salts drug candidate, which is currently being evaluated in clinical studies for the treatment of Iron Deficiencies.
Product Name : Undisclosed
Product Type : Vitamins/Minerals/Inorganic Salts
Upfront Cash : Inapplicable
January 11, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
BGE-117 is a drug candidate, which is currently being evaluated in phase II clinical studies for the treatment of Anemia, Posthemorrhagic.
Lead Product(s): BGE-117,Ferrous Sulfate
Therapeutic Area: Hematology Brand Name: Undisclosed
Study Phase: Phase IIProduct Type: Undisclosed
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable December 10, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : BGE-117,Ferrous Sulfate
Therapeutic Area : Hematology
Highest Development Status : Phase II
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : BGE-117 is a drug candidate, which is currently being evaluated in phase II clinical studies for the treatment of Anemia, Posthemorrhagic.
Product Name : Undisclosed
Product Type : Undisclosed
Upfront Cash : Inapplicable
December 10, 2021

Details:
Ferrous Sulfate is a Vitamins/Minerals/Inorganic Salts drug candidate, which is currently being evaluated in clinical studies for the treatment of Premenopause.
Lead Product(s): Ferrous Sulfate,Inapplicable
Therapeutic Area: Obstetrics/Gynecology (Women’s Health) Brand Name: Undisclosed
Study Phase: UndisclosedProduct Type: Vitamins/Minerals/Inorganic Salts
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable March 02, 2021

Lead Product(s) : Ferrous Sulfate,Inapplicable
Therapeutic Area : Obstetrics/Gynecology (Women’s Health)
Highest Development Status : Undisclosed
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : Ferrous Sulfate is a Vitamins/Minerals/Inorganic Salts drug candidate, which is currently being evaluated in clinical studies for the treatment of Premenopause.
Product Name : Undisclosed
Product Type : Vitamins/Minerals/Inorganic Salts
Upfront Cash : Inapplicable
March 02, 2021

Details:
Ferrous Sulphate is a Other Small Molecule drug candidate, which is currently being evaluated in clinical studies for the treatment of Iron Deficiencies.
Lead Product(s): Ferrous Sulfate,Curcumin
Therapeutic Area: Hematology Brand Name: Undisclosed
Study Phase: UndisclosedProduct Type: Miscellaneous
Sponsor: Gencor Pacific Group
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable July 10, 2020

Lead Product(s) : Ferrous Sulfate,Curcumin
Therapeutic Area : Hematology
Highest Development Status : Undisclosed
Partner/Sponsor/Collaborator : Gencor Pacific Group
Deal Size : Inapplicable
Deal Type : Inapplicable
Effect of Ferrous iROn and cUrcumin sTatus on Inflammatory and Neurotrophic markErs
Details : Ferrous Sulphate is a Other Small Molecule drug candidate, which is currently being evaluated in clinical studies for the treatment of Iron Deficiencies.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
July 10, 2020

Details:
Ferumoxytol is a Vitamins/Minerals/Inorganic Salts drug candidate, which is currently being evaluated in phase III clinical studies for the treatment of Anemia, Iron-Deficiency.
Lead Product(s): Ferumoxytol,Sodium Chloride,Ferrous Sulfate,Ascorbic Acid
Therapeutic Area: Hematology Brand Name: Undisclosed
Study Phase: Phase IIIProduct Type: Vitamins/Minerals/Inorganic Salts
Sponsor: AMAG Pharmaceuticals
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable February 13, 2020

Lead Product(s) : Ferumoxytol,Sodium Chloride,Ferrous Sulfate,Ascorbic Acid
Therapeutic Area : Hematology
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : AMAG Pharmaceuticals
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : Ferumoxytol is a Vitamins/Minerals/Inorganic Salts drug candidate, which is currently being evaluated in phase III clinical studies for the treatment of Anemia, Iron-Deficiency.
Product Name : Undisclosed
Product Type : Vitamins/Minerals/Inorganic Salts
Upfront Cash : Inapplicable
February 13, 2020

Details:
Iron is a Vitamins/Minerals/Inorganic Salts drug candidate, which is currently being evaluated in clinical studies for the treatment of undefined medical condition.
Lead Product(s): Iron,Prebiotic Mixture,Fefum,Ferrous Sulfate
Therapeutic Area: Undisclosed Brand Name: Undisclosed
Study Phase: UndisclosedProduct Type: Vitamins/Minerals/Inorganic Salts
Sponsor: Danone Nutricia Research
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable March 28, 2019

Lead Product(s) : Iron,Prebiotic Mixture,Fefum,Ferrous Sulfate
Therapeutic Area : Undisclosed
Highest Development Status : Undisclosed
Partner/Sponsor/Collaborator : Danone Nutricia Research
Deal Size : Inapplicable
Deal Type : Inapplicable
Iron Absorption From a Wheat-based Instant Cereal:Gut and Stable Isotope Studies in Kenyan Infants
Details : Iron is a Vitamins/Minerals/Inorganic Salts drug candidate, which is currently being evaluated in clinical studies for the treatment of undefined medical condition.
Product Name : Undisclosed
Product Type : Vitamins/Minerals/Inorganic Salts
Upfront Cash : Inapplicable
March 28, 2019

Details:
Ferumoxytol is a Vitamins/Minerals/Inorganic Salts drug candidate, which is currently being evaluated in clinical studies for the treatment of Post-Bariatric, Anemia, Iron-Deficiency.
Lead Product(s): Ferumoxytol,Ascorbic Acid,Ferrous Sulfate,Sodium Chloride
Therapeutic Area: Hematology Brand Name: Undisclosed
Study Phase: UndisclosedProduct Type: Vitamins/Minerals/Inorganic Salts
Sponsor: AMAG Pharmaceuticals
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable December 17, 2018

Lead Product(s) : Ferumoxytol,Ascorbic Acid,Ferrous Sulfate,Sodium Chloride
Therapeutic Area : Hematology
Highest Development Status : Undisclosed
Partner/Sponsor/Collaborator : AMAG Pharmaceuticals
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : Ferumoxytol is a Vitamins/Minerals/Inorganic Salts drug candidate, which is currently being evaluated in clinical studies for the treatment of Post-Bariatric, Anemia, Iron-Deficiency.
Product Name : Undisclosed
Product Type : Vitamins/Minerals/Inorganic Salts
Upfront Cash : Inapplicable
December 17, 2018

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Related Excipient Companies

Dosage Form : Tablet
Grade : Oral & Parenteral
Application : Surfactant & Foaming Agents
Excipient Details : Polysorbate 80 is widely used as a surfactant, emulsifier, solubilizer, and stabilizer.
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
70
PharmaCompass offers a list of Ferrous Sulfate API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Ferrous Sulfate manufacturer or Ferrous Sulfate supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Ferrous Sulfate manufacturer or Ferrous Sulfate supplier.
PharmaCompass also assists you with knowing the Ferrous Sulfate API Price utilized in the formulation of products. Ferrous Sulfate API Price is not always fixed or binding as the Ferrous Sulfate Price is obtained through a variety of data sources. The Ferrous Sulfate Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Ferrous sulfate, dried manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Ferrous sulfate, dried, including repackagers and relabelers. The FDA regulates Ferrous sulfate, dried manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Ferrous sulfate, dried API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Ferrous sulfate, dried manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Ferrous sulfate, dried supplier is an individual or a company that provides Ferrous sulfate, dried active pharmaceutical ingredient (API) or Ferrous sulfate, dried finished formulations upon request. The Ferrous sulfate, dried suppliers may include Ferrous sulfate, dried API manufacturers, exporters, distributors and traders.
click here to find a list of Ferrous sulfate, dried suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Ferrous sulfate, dried DMF (Drug Master File) is a document detailing the whole manufacturing process of Ferrous sulfate, dried active pharmaceutical ingredient (API) in detail. Different forms of Ferrous sulfate, dried DMFs exist exist since differing nations have different regulations, such as Ferrous sulfate, dried USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Ferrous sulfate, dried DMF submitted to regulatory agencies in the US is known as a USDMF. Ferrous sulfate, dried USDMF includes data on Ferrous sulfate, dried's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Ferrous sulfate, dried USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Ferrous sulfate, dried suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Ferrous sulfate, dried Drug Master File in Japan (Ferrous sulfate, dried JDMF) empowers Ferrous sulfate, dried API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Ferrous sulfate, dried JDMF during the approval evaluation for pharmaceutical products. At the time of Ferrous sulfate, dried JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Ferrous sulfate, dried suppliers with JDMF on PharmaCompass.
A Ferrous sulfate, dried CEP of the European Pharmacopoeia monograph is often referred to as a Ferrous sulfate, dried Certificate of Suitability (COS). The purpose of a Ferrous sulfate, dried CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Ferrous sulfate, dried EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Ferrous sulfate, dried to their clients by showing that a Ferrous sulfate, dried CEP has been issued for it. The manufacturer submits a Ferrous sulfate, dried CEP (COS) as part of the market authorization procedure, and it takes on the role of a Ferrous sulfate, dried CEP holder for the record. Additionally, the data presented in the Ferrous sulfate, dried CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Ferrous sulfate, dried DMF.
A Ferrous sulfate, dried CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Ferrous sulfate, dried CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Ferrous sulfate, dried suppliers with CEP (COS) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Ferrous sulfate, dried as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Ferrous sulfate, dried API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Ferrous sulfate, dried as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Ferrous sulfate, dried and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Ferrous sulfate, dried NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Ferrous sulfate, dried suppliers with NDC on PharmaCompass.
Ferrous sulfate, dried Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Ferrous sulfate, dried GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Ferrous sulfate, dried GMP manufacturer or Ferrous sulfate, dried GMP API supplier for your needs.
A Ferrous sulfate, dried CoA (Certificate of Analysis) is a formal document that attests to Ferrous sulfate, dried's compliance with Ferrous sulfate, dried specifications and serves as a tool for batch-level quality control.
Ferrous sulfate, dried CoA mostly includes findings from lab analyses of a specific batch. For each Ferrous sulfate, dried CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Ferrous sulfate, dried may be tested according to a variety of international standards, such as European Pharmacopoeia (Ferrous sulfate, dried EP), Ferrous sulfate, dried JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Ferrous sulfate, dried USP).

