



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0

USA (Orange Book)

Europe

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
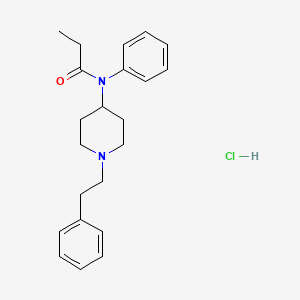

1. Fentanyl Hcl
2. 1443-54-5
3. 59h156xy46
4. Fentanyl Hydrochloride (jan)
5. Fentanyl Hydrochloride [jan]
6. N-phenyl-n-[1-(2-phenylethyl)piperidin-4-yl]propanamide;hydrochloride
7. Unii-59h156xy46
8. Ionsys (tn)
9. N-(1-phenethyl-4-piperidyl)propionanilide Hydrochloride
10. Mls002320669
11. Schembl242876
12. Chembl1201159
13. Dtxsid90162666
14. Propionanilide, N-(1-phenethyl-4-piperidyl)-, Hydrochloride
15. Fentanyl Hydrochloride [mart.]
16. Fentanyl Hydrochloride [who-dd]
17. Fentanyl Hydrochloride [ema Epar]
18. Smr001338815
19. Fentanyl Hydrochloride [orange Book]
20. D10811
21. Q27261701
22. Propanamide, N-phenyl-n-(1-(2-phenylethyl)-4-piperidinyl)-, Monohydrochloride
| Molecular Weight | 372.9 g/mol |
|---|---|
| Molecular Formula | C22H29ClN2O |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 6 |
| Exact Mass | 372.1968412 g/mol |
| Monoisotopic Mass | 372.1968412 g/mol |
| Topological Polar Surface Area | 23.6 Ų |
| Heavy Atom Count | 26 |
| Formal Charge | 0 |
| Complexity | 391 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Ionsys is indicated for the management of acute moderate to severe post-operative pain in adult patients.
Management of acute moderate to severe post-operative pain for use in a hospital setting only
Treatment of acute pain
N02AB03
N02AB03


