



API Suppliers

US DMFs Filed

CEP/COS Certifications

JDMFs Filed
Other Certificates
Other Suppliers
0

USA (Orange Book)

Europe

Canada

Australia
0

South Africa
Uploaded Dossiers
U.S. Medicaid
Annual Reports
0

1. Duragesic
2. Durogesic
3. Fentanest
4. Fentanyl
5. Fentora
6. Phentanyl
7. R 4263
8. R-4263
9. R4263
10. Sublimaze
11. Transmucosal Oral Fentanyl Citrate
1. 990-73-8
2. Leptanal
3. Phentanyl Citrate
4. Actiq
5. Fentanyl Dihydrogen Citrate
6. Abstral
7. Fentanyl Citrate Salt
8. Lazanda
9. Onsolis
10. Mcn-jr-4263-49
11. Instanyl
12. Fentanyl Buccal
13. Fentanyl Citrate Cii
14. Mun5lyg46h
15. Oralet
16. N-(1-phenethyl-4-piperidyl)propionanilide Citrate
17. N-(1-phenethyl-4-piperidyl)propionanilide Citrate (1:1)
18. N-(1-phenethyl-4-piperidyl)propionanilide Dihydrogen Citrate
19. Chebi:31602
20. Fentaz
21. N-(1-phenethyl-4-piperidinyl)propionanilide Dihydrogen Citrate
22. R-4263
23. Kw-2246
24. Propanamide, N-phenyl-n-(1-(2-phenylethyl)-4-piperidinyl)-, 2-hydroxy-1,2,3-propanetricarboxylate (1:1)
25. N-(1-phenethylpiperidin-4-yl)-n-phenylpropionamide Citrate
26. Rapinyl
27. 2-hydroxypropane-1,2,3-tricarboxylic Acid;n-phenyl-n-[1-(2-phenylethyl)piperidin-4-yl]propanamide
28. Mcn-jr 4263
29. Sublimaze Preservative Free
30. Einecs 213-588-0
31. Unii-mun5lyg46h
32. Fentanyl Citrate Preservative Free
33. R 5240
34. Fentanyl Monocitrate
35. Abstral (tn)
36. Fentora (tn)
37. Lazanda (tn)
38. Oralet (tn)
39. Fentanyl Citrate [usan:usp:ban:jan]
40. Propionanilide, N-(1-phenethyl-4-piperidyl)-, Citrate (1:1)
41. Chembl688
42. Schembl40733
43. Fentanyl Citrate [mi]
44. Fentanyl Citrate [jan]
45. Fentanyl Citrate (jp17/usp)
46. Fentanyl Citrate [usan]
47. Fentanyl Citrate [vandf]
48. Dtxsid80243933
49. Fentanyl Citrate [mart.]
50. Fentanyl Citrate [who-dd]
51. Fentanyl Citrate [ema Epar]
52. Fentanyl Citrate [green Book]
53. Akos024457504
54. Fentanyl Citrate [orange Book]
55. Fentanyl Citrate Cii [usp-rs]
56. Fentanyl Citrate [ep Monograph]
57. Fentanyl Citrate [usp Monograph]
58. Innovar Component Fentanyl Citrate
59. B5403
60. Fentanyl Citrate Component Of Innovar
61. D01399
62. 990f738
63. Q27104201
64. N-phenyl-n-[1-(2-phenylethyl)-4-piperidyl]propanamide Citrate
65. Fentanyl Citrate, European Pharmacopoeia (ep) Reference Standard
66. N-phenyl-n-[1-(2-phenylethyl)-4-piperidinyl]propanamide Citrate
67. Fentanyl Citrate, United States Pharmacopeia (usp) Reference Standard
68. Fentanyl Citrate Salt Solution, Drug Standard, 100 Mug/ml In Methanol: Tert-butanol (3:2)
69. N-(1-phenethylpiperidin-4-yl)-n-phenylpropionamide 2-hydroxypropane-1,2,3-tricarboxylate
70. N-phenyl-n-[1-(2-phenylethyl)piperidin-4-yl]propanamide 2-hydroxypropane-1,2,3-tricarboxylate
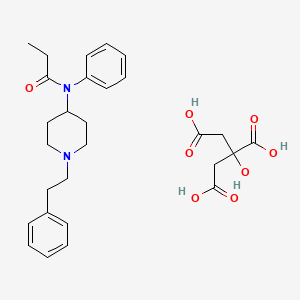
| Molecular Weight | 528.6 g/mol |
|---|---|
| Molecular Formula | C28H36N2O8 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 11 |
| Exact Mass | 528.24716611 g/mol |
| Monoisotopic Mass | 528.24716611 g/mol |
| Topological Polar Surface Area | 156 Ų |
| Heavy Atom Count | 38 |
| Formal Charge | 0 |
| Complexity | 618 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 8 | |
|---|---|
| Drug Name | Abstral |
| PubMed Health | Fentanyl |
| Drug Classes | Analgesic, Anesthetic Adjunct |
| Drug Label | ABSTRAL (fentanyl) sublingual tablet is a solid formulation of fentanyl citrate, a potent opioid analgesic intended for oral sublingual administration. ABSTRAL is formulated as a white tablet available in six strengths, distinguishable by the shape o... |
| Active Ingredient | Fentanyl citrate |
| Dosage Form | Tablet |
| Route | Sublingual |
| Strength | eq 0.1mg base; eq 0.8mg base; eq 0.3mg base; eq 0.6mg base; eq 0.4mg base; eq 0.2mg base |
| Market Status | Prescription |
| Company | Galena Biopharma |
| 2 of 8 | |
|---|---|
| Drug Name | Fentanyl citrate |
| Drug Label | Oral transmucosal fentanyl citrate is a solid formulation of fentanyl citrate, a potent opioid analgesic, intended for oral transmucosal administration. Oral transmucosal fentanyl citrate is formulated as a white to off-white solid drug matrix on a h... |
| Active Ingredient | Fentanyl citrate |
| Dosage Form | Injectable; Troche/lozenge |
| Route | Transmucosal; Injection |
| Strength | eq 0.05mg base/ml; eq 0.8mg base; eq 1.6mg base; eq 0.6mg base; eq 1.2mg base; eq 0.4mg base; eq 0.2mg base |
| Market Status | Prescription |
| Company | Hospira; Mallinckrodt; Par Pharm |
| 3 of 8 | |
|---|---|
| Drug Name | Fentanyl citrate preservative free |
| Active Ingredient | Fentanyl citrate |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 0.05mg base/ml |
| Market Status | Prescription |
| Company | Hospira; Hikma Maple |
| 4 of 8 | |
|---|---|
| Drug Name | Sublimaze preservative free |
| Active Ingredient | Fentanyl citrate |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 0.05mg base/ml |
| Market Status | Prescription |
| Company | Akorn |
| 5 of 8 | |
|---|---|
| Drug Name | Abstral |
| PubMed Health | Fentanyl |
| Drug Classes | Analgesic, Anesthetic Adjunct |
| Drug Label | ABSTRAL (fentanyl) sublingual tablet is a solid formulation of fentanyl citrate, a potent opioid analgesic intended for oral sublingual administration. ABSTRAL is formulated as a white tablet available in six strengths, distinguishable by the shape o... |
| Active Ingredient | Fentanyl citrate |
| Dosage Form | Tablet |
| Route | Sublingual |
| Strength | eq 0.1mg base; eq 0.8mg base; eq 0.3mg base; eq 0.6mg base; eq 0.4mg base; eq 0.2mg base |
| Market Status | Prescription |
| Company | Galena Biopharma |
| 6 of 8 | |
|---|---|
| Drug Name | Fentanyl citrate |
| Drug Label | Oral transmucosal fentanyl citrate is a solid formulation of fentanyl citrate, a potent opioid analgesic, intended for oral transmucosal administration. Oral transmucosal fentanyl citrate is formulated as a white to off-white solid drug matrix on a h... |
| Active Ingredient | Fentanyl citrate |
| Dosage Form | Injectable; Troche/lozenge |
| Route | Transmucosal; Injection |
| Strength | eq 0.05mg base/ml; eq 0.8mg base; eq 1.6mg base; eq 0.6mg base; eq 1.2mg base; eq 0.4mg base; eq 0.2mg base |
| Market Status | Prescription |
| Company | Hospira; Mallinckrodt; Par Pharm |
| 7 of 8 | |
|---|---|
| Drug Name | Fentanyl citrate preservative free |
| Active Ingredient | Fentanyl citrate |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 0.05mg base/ml |
| Market Status | Prescription |
| Company | Hospira; Hikma Maple |
| 8 of 8 | |
|---|---|
| Drug Name | Sublimaze preservative free |
| Active Ingredient | Fentanyl citrate |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 0.05mg base/ml |
| Market Status | Prescription |
| Company | Akorn |
Instanyl is indicated for the management of breakthrough pain in adults already receiving maintenance opioid therapy for chronic cancer pain. Breakthrough pain is a transitory exacerbation of pain that occurs on a background of otherwise controlled persistent pain.
Patients receiving maintenance opioid therapy are those who are taking at least 60 mg of oral morphine daily, at least 25 micrograms of transdermal fentanyl per hour, at least 30 mg oxycodone daily, at least 8 mg of oral hydromorphone daily or an equianalgesic dose of another opioid for a week or longer.
Treatment of acute pain, Prevention of acute pain
Acute pain
Analgesics, Opioid
Compounds with activity like OPIATE ALKALOIDS, acting at OPIOID RECEPTORS. Properties include induction of ANALGESIA or NARCOSIS. (See all compounds classified as Analgesics, Opioid.)
Anesthetics, Intravenous
Ultrashort-acting anesthetics that are used for induction. Loss of consciousness is rapid and induction is pleasant, but there is no muscle relaxation and reflexes frequently are not reduced adequately. Repeated administration results in accumulation and prolongs the recovery time. Since these agents have little if any analgesic activity, they are seldom used alone except in brief minor procedures. (From AMA Drug Evaluations Annual, 1994, p174) (See all compounds classified as Anesthetics, Intravenous.)
Narcotics
Agents that induce NARCOSIS. Narcotics include agents that cause somnolence or induced sleep (STUPOR); natural or synthetic derivatives of OPIUM or MORPHINE or any substance that has such effects. They are potent inducers of ANALGESIA and OPIOID-RELATED DISORDERS. (See all compounds classified as Narcotics.)
Adjuvants, Anesthesia
Agents that are administered in association with anesthetics to increase effectiveness, improve delivery, or decrease required dosage. (See all compounds classified as Adjuvants, Anesthesia.)
N02AB03








