
Synopsis
Synopsis
0
VMF
0
South Africa

0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
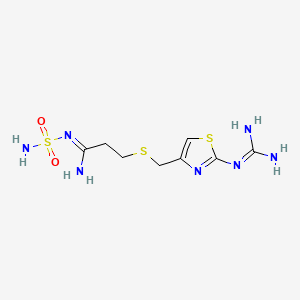

1. Famotidine
2. Famotidine Hydrochloride
3. Mk 208
4. Mk-208
5. Mk208
6. Ym 11170
7. Ym-11170
8. Ym11170
1. Famotidine
2. 76824-35-6
3. Gastridin
4. Famodil
5. Pepdine
6. Pepdul
7. Pepcidine
8. Famoxal
9. Ganor
10. Pepcidac
11. Amfamox
12. Gastropen
13. Famosan
14. Fluxid
15. Gaster
16. Lecedil
17. Motiax
18. Muclox
19. Pepcid Ac
20. Quamatel
21. Ym-11170
22. Pepcid Rpd
23. Fadul
24. Mk-208
25. Famotidine (pepcid)
26. 3-[({2-[(diaminomethylidene)amino]-1,3-thiazol-4-yl}methyl)sulfanyl]-n-sulfamoylpropanimidamide
27. L 643341
28. Mk 208
29. Chebi:4975
30. Ym 11170
31. Nsc-757810
32. 3-(((2-((diaminomethylene)amino)thiazol-4-yl)methyl)thio)-n'-sulfamoylpropanimidamide
33. Xufqphaneapemj-uhfffaoysa-
34. 3-(((2-guanidinothiazol-4-yl)methyl)thio)-n-sulfamoylpropanimidamide
35. (1-amino-3-(((2-((diaminomethylene)amino)-4-thiazolyl)methyl)thio)propylidene)sulfamide
36. Pepcid Ac Gelcaps
37. Smr000058961
38. Pepcid (tn)
39. Chembl902
40. 3-[[[2-[(aminoiminomethyl)amino]-4-thiazolyl]methyl]thio]-n-(aminosulfonyl)propanimidamide
41. Sr-05000001440
42. 5qzo15j2z8
43. Ncgc00015446-05
44. Fo9
45. Propanimidamide, N'-(aminosulfonyl)-3-(((2-((diaminomethylene)amino)-4-thiazolyl)methyl)thio)-
46. Prestwick_212
47. Cas-76824-35-6
48. Fluxid (tn)
49. Mfcd00079297
50. Hs-0054
51. Ym-1170
52. Famotidine [mi]
53. 3-(2-guanidinothiazol-4-ylmethylthio)-n1-sulfamoylpropionamide
54. Famotidine [inn]
55. Famotidine [jan]
56. Prestwick2_000104
57. Prestwick3_000104
58. Famotidine [hsdb]
59. Famotidine [usan]
60. Lopac-f-6889
61. F0530
62. Schembl972
63. Schembl974
64. Famotidine [vandf]
65. F 6889
66. Famotidine [mart.]
67. Famotidine [usp-rs]
68. Famotidine [who-dd]
69. N'-(aminosulfonyl)-3-([2-(diaminomethyleneamino)-4-thiazolyl]methylthio)propanamidine
70. Lopac0_000497
71. Bspbio_000088
72. Mls000028583
73. Mls000758205
74. Mls001423994
75. Bidd:gt0759
76. Bpbio1_000098
77. Famotidine (jp17/usp/inn)
78. Famotidine [orange Book]
79. Bdbm22891
80. Cid_5702160
81. Famotidine [ep Monograph]
82. Famotidine [usp Impurity]
83. Famotidine [usp Monograph]
84. Hms1568e10
85. Hms2051a07
86. Hms2089i12
87. Hms2095e10
88. Hms2231i22
89. Hms3261d15
90. Hms3712e10
91. Hms3884j03
92. Hy-b0377
93. Tox21_500497
94. Bdbm50103514
95. Pdsp1_000398
96. Pdsp2_000396
97. S2078
98. Stk527689
99. Akos005460541
100. Akos015994617
101. Ccg-100767
102. Ccg-220104
103. Ccg-221801
104. Nc00017
105. Sdccgsbi-0050481.p004
106. 3-[[2-(diaminomethylideneamino)-1,3-thiazol-4-yl]methylsulfanyl]-n'-sulfamoylpro
107. Propanimidamide, 3-[[[2-[aminoiminomethyl)amino]-4-thiazoyl]methyl]thio]-n-(aminosulfonyl)
108. Ncgc00015446-01
109. Ncgc00015446-02
110. Ncgc00015446-03
111. Ncgc00015446-04
112. Ncgc00015446-06
113. Ncgc00015446-08
114. Ncgc00018276-01
115. Ncgc00018276-02
116. Ncgc00093899-01
117. Ncgc00093899-02
118. Ncgc00093899-03
119. Ncgc00188952-01
120. Ncgc00261182-01
121. (1z)-3-[({2-[(diaminomethylidene)amino]-1,3-thiazol-4-yl}methyl)sulfanyl]-n'-sulfamoylpropanimidamide
122. (1z)-n'-(aminosulfonyl)-3-[({2-[(diaminomethylidene)amino]-1,3-thiazol-4-yl}methyl)thio]propanimidamide
123. Ac-11713
124. Ac-31723
125. Pepcid Complete Component Famotidine
126. Sbi-0050481.p003
127. Eu-0100497
128. Famotidine Component Of Pepcid Complete
129. D00318
130. Ab00383032_11
131. 824f356
132. A838850
133. A899959
134. L003830
135. L013386
136. Sr-01000075883
137. Sr-01000075883-1
138. Sr-05000001440-1
139. Sr-05000001440-2
140. Famotidine, British Pharmacopoeia (bp) Reference Standard
141. Famotidine, European Pharmacopoeia (ep) Reference Standard
142. Famotidine, United States Pharmacopeia (usp) Reference Standard
143. 3-[(2-guanidinothiazol-4-yl)methylsulfanyl]-n-sulfamoyl-propanamidine
144. Famotidine, Pharmaceutical Secondary Standard; Certified Reference Material
145. 3-((((2-(diaminomethylene)amino)-4-thiazolyl)methyl)thio)-n-sulfamoylpropionamidine
146. 3-(2-(diaminomethyleneamino)-1,3-thiazol-4-yl)methylthio)-n'- Sulfamoylpropionamidine
147. 3-[2-(diaminomethyleneamino] Thiazol-4-ylmethylthio]-n-sulphamoyl Propionamidine
148. Famotidine For System Suitability, European Pharmacopoeia (ep) Reference Standard
149. 3-[({2-[(diaminomethylene)amino]-1,3-thiazol-4-yl}methyl)sulfanyl]-n'-sulfamoylpropanimidamide
150. 3-[({2-[(diaminomethylidene)amino]-1,3-thiazol-4-yl}methyl)sulfanyl]-n''-sulfamoylpropanimidamide
| Molecular Weight | 337.5 g/mol |
|---|---|
| Molecular Formula | C8H15N7O2S3 |
| XLogP3 | -0.6 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 7 |
| Exact Mass | 337.04493627 g/mol |
| Monoisotopic Mass | 337.04493627 g/mol |
| Topological Polar Surface Area | 238 Ų |
| Heavy Atom Count | 20 |
| Formal Charge | 0 |
| Complexity | 469 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 10 | |
|---|---|
| Drug Name | Famotidine |
| Active Ingredient | Famotidine |
| Dosage Form | Injectable; Tablet; Tablet, chewable; For suspension; Suspension |
| Route | injection; Injection; Oral |
| Strength | 40mg/5ml; 10mg; 10mg/ml; 40mg; 20mg |
| Market Status | Tentative Approval; Over the Counter; Prescription |
| Company | Navinta; Ranbaxy; Wockhardt; Bedford; Ivax Sub Teva Pharms; Marsam Pharms; Fresenius Kabi Usa; Hi-tech Pharma; Hikma Maple; Teva; Apotex; Perrigo; Alembic Pharms; Lupin; Dr Reddys Labs; Carlsbad; Agila Speclts; Mylan; Novel Labs |
| 2 of 10 | |
|---|---|
| Drug Name | Famotidine preservative free |
| PubMed Health | Famotidine |
| Drug Classes | Antiulcer, Gastric Acid Secretion Inhibitor |
| Drug Label | The active ingredient in PEPCID (famotidine) is a histamine H2-receptor antagonist. Famotidine is N'-(aminosulfonyl)-3-[[[2-[(diaminomethylene)amino]-4-thiazolyl]methyl]thio]propanimidamide. The empirical formula of famotidine is C8H15N7O2S3 and it.. |
| Active Ingredient | Famotidine |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 10mg/ml |
| Market Status | Prescription |
| Company | Bedford; Fresenius Kabi Usa; Hikma Maple; Bedford Labs; Agila Speclts |
| 3 of 10 | |
|---|---|
| Drug Name | Famotidine preservative free in plastic container |
| PubMed Health | Famotidine (By mouth) |
| Drug Classes | Antiulcer, Gastric Acid Secretion Inhibitor |
| Active Ingredient | Famotidine |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 0.4mg/ml |
| Market Status | Prescription |
| Company | Baxter Hlthcare |
| 4 of 10 | |
|---|---|
| Drug Name | Pepcid |
| PubMed Health | Famotidine |
| Drug Classes | Antiulcer, Gastric Acid Secretion Inhibitor |
| Drug Label | The active ingredient in Famotidine Injection, USP is a histamine H2-receptor antagonist. Famotidine is [1-Amino-3-[[[2-[(diaminomethylene)amino]-4-thiazolyl]methyl]thio]propylidene] sulfamide. Its structural formula is:C8H15N7O2S3 MW 337.4... |
| Active Ingredient | Famotidine |
| Dosage Form | Tablet; For suspension |
| Route | Oral |
| Strength | 40mg/5ml; 40mg; 20mg |
| Market Status | Prescription |
| Company | Marathon Pharms; Salix Pharms |
| 5 of 10 | |
|---|---|
| Drug Name | Pepcid ac |
| Active Ingredient | Famotidine |
| Dosage Form | Tablet; Tablet, chewable |
| Route | Oral |
| Strength | 10mg; 20mg |
| Market Status | Over the Counter |
| Company | Mcneil Cons |
| 6 of 10 | |
|---|---|
| Drug Name | Famotidine |
| Active Ingredient | Famotidine |
| Dosage Form | Injectable; Tablet; Tablet, chewable; For suspension; Suspension |
| Route | injection; Injection; Oral |
| Strength | 40mg/5ml; 10mg; 10mg/ml; 40mg; 20mg |
| Market Status | Tentative Approval; Over the Counter; Prescription |
| Company | Navinta; Ranbaxy; Wockhardt; Bedford; Ivax Sub Teva Pharms; Marsam Pharms; Fresenius Kabi Usa; Hi-tech Pharma; Hikma Maple; Teva; Apotex; Perrigo; Alembic Pharms; Lupin; Dr Reddys Labs; Carlsbad; Agila Speclts; Mylan; Novel Labs |
| 7 of 10 | |
|---|---|
| Drug Name | Famotidine preservative free |
| PubMed Health | Famotidine |
| Drug Classes | Antiulcer, Gastric Acid Secretion Inhibitor |
| Drug Label | The active ingredient in PEPCID (famotidine) is a histamine H2-receptor antagonist. Famotidine is N'-(aminosulfonyl)-3-[[[2-[(diaminomethylene)amino]-4-thiazolyl]methyl]thio]propanimidamide. The empirical formula of famotidine is C8H15N7O2S3 and it.. |
| Active Ingredient | Famotidine |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 10mg/ml |
| Market Status | Prescription |
| Company | Bedford; Fresenius Kabi Usa; Hikma Maple; Bedford Labs; Agila Speclts |
| 8 of 10 | |
|---|---|
| Drug Name | Famotidine preservative free in plastic container |
| PubMed Health | Famotidine (By mouth) |
| Drug Classes | Antiulcer, Gastric Acid Secretion Inhibitor |
| Active Ingredient | Famotidine |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 0.4mg/ml |
| Market Status | Prescription |
| Company | Baxter Hlthcare |
| 9 of 10 | |
|---|---|
| Drug Name | Pepcid |
| PubMed Health | Famotidine |
| Drug Classes | Antiulcer, Gastric Acid Secretion Inhibitor |
| Drug Label | The active ingredient in Famotidine Injection, USP is a histamine H2-receptor antagonist. Famotidine is [1-Amino-3-[[[2-[(diaminomethylene)amino]-4-thiazolyl]methyl]thio]propylidene] sulfamide. Its structural formula is:C8H15N7O2S3 MW 337.4... |
| Active Ingredient | Famotidine |
| Dosage Form | Tablet; For suspension |
| Route | Oral |
| Strength | 40mg/5ml; 40mg; 20mg |
| Market Status | Prescription |
| Company | Marathon Pharms; Salix Pharms |
| 10 of 10 | |
|---|---|
| Drug Name | Pepcid ac |
| Active Ingredient | Famotidine |
| Dosage Form | Tablet; Tablet, chewable |
| Route | Oral |
| Strength | 10mg; 20mg |
| Market Status | Over the Counter |
| Company | Mcneil Cons |
Anti-Ulcer Agents; Histamine H2 Antagonists
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Famotidine is currently the drug of choice for initial treatment and maintenance therapy in most patients with uncomplicated gastric or duodenal ulcer. ... A single bedtime dose of famotidine 40 mg is as efficatious as previously recommended multidose regimens and increases compliance.
American Medical Association, Council on Drugs. AMA Drug Evaluations Annual 1994. Chicago, IL: American Medical Association, 1994., p. 892
Histamine H2-receptor antagonists are indicated in the short-term treatment of active duodenal ulcer. They are also indicated (at reduce dosage) in the prevention of duodenal ulcer recurrence in selected patients. /Histamine H2-receptor antagonists; Included in US product labeling/
USP Convention. USPDI - Drug Information for the Health Care Professional. 16th ed. Volume I. Rockville, MD: U.S. Pharmaceutical Convention, Inc. 1996 (Plus updates)., p. 1611
Famotidine ... /is/ indicated in the short-term treatment of active benign gastric ulcer. /Included in US product labeling/
USP Convention. USPDI - Drug Information for the Health Care Professional. 16th ed. Volume I. Rockville, MD: U.S. Pharmaceutical Convention, Inc. 1996 (Plus updates)., p. 1611
For more Therapeutic Uses (Complete) data for FAMOTIDINE (12 total), please visit the HSDB record page.
Although appropriate studies on the relationship of age to the effects of these medicines /cimetidine, famotidine, and ranitidine/ have not been performed in the geriatric population, no geriatrics-specific problems have been documented to date. However, confusion is more likely to occur in elderly patients with impaired hepatic or renal function.
USP Convention. USPDI - Drug Information for the Health Care Professional. 16th ed. Volume I. Rockville, MD: U.S. Pharmaceutical Convention, Inc. 1996 (Plus updates)., p. 1613
Adverse nervous system effects (eg, headache, dizziness) and GI effects (eg, constipation, diarrhea) occur most frequently during famotidine therapy. Although adverse effects of the drug generally are not severe, discontinuance of famotidine therapy has been necessary in up to 14% of patients. Adverse effects generally are similar when famotidine is administered orally or IV.
McEvoy G.K. (ed.). American Hospital Formulary Service-Drug Information 96. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1996 (Plus Supplements)., p. 2151
Fever, hypertension, flushing, musculoskeletal pain, arthralgia, and tinnitus have been reported in 1% or less of patients receiving famotidine, but a causal relationship to the drug has not been established in many cases. An acute episode of gout occurred in one patient during therapy with the drug.
McEvoy G.K. (ed.). American Hospital Formulary Service-Drug Information 96. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1996 (Plus Supplements)., p. 2151
Leukocytosis, leukopenia, neutropenia, pancytopenia, agranulocytosis, eosinophilia, prolonged erythrocyte sedimentation rate (ESR), and thrombocytopenia have occurred rarely in patients receiving famotidine. Changes in serum protein or cholesterol concentrations also have occurred.
McEvoy G.K. (ed.). American Hospital Formulary Service-Drug Information 96. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1996 (Plus Supplements)., p. 2151
For more Drug Warnings (Complete) data for FAMOTIDINE (10 total), please visit the HSDB record page.
Anti-Ulcer Agents
Various agents with different action mechanisms used to treat or ameliorate PEPTIC ULCER or irritation of the gastrointestinal tract. This has included ANTIBIOTICS to treat HELICOBACTER INFECTIONS; HISTAMINE H2 ANTAGONISTS to reduce GASTRIC ACID secretion; and ANTACIDS for symptomatic relief. (See all compounds classified as Anti-Ulcer Agents.)
Histamine H2 Antagonists
Drugs that selectively bind to but do not activate histamine H2 receptors, thereby blocking the actions of histamine. Their clinically most important action is the inhibition of acid secretion in the treatment of gastrointestinal ulcers. Smooth muscle may also be affected. Some drugs in this class have strong effects in the central nervous system, but these actions are not well understood. (See all compounds classified as Histamine H2 Antagonists.)
A - Alimentary tract and metabolism
A02 - Drugs for acid related disorders
A02B - Drugs for peptic ulcer and gastro-oesophageal reflux disease (gord)
A02BA - H2-receptor antagonists
A02BA03 - Famotidine
All H2-receptor antagonists are distributed in breast milk and cerebral spinal fluid. /Histamine H2-receptor antagonists/
USP Convention. USPDI - Drug Information for the Health Care Professional. 16th ed. Volume I. Rockville, MD: U.S. Pharmaceutical Convention, Inc. 1996 (Plus updates)., p. 1612
Distribution of famotidine into human body tissues and fluids has not been fully characterized. The apparent volume of distribution of the drug is reported to be 1.1-1.4 l/kg in adults and does not appear to be altered substantially in patients with renal dysfunction. Following oral or IV administration in rats, famotidine is widely distributed, appearing in highest concentrations in the kidney, liver, pancreas, and submandibular gland. The drug is 15-20% protein bound.
McEvoy G.K. (ed.). American Hospital Formulary Service-Drug Information 96. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1996 (Plus Supplements)., p. 2149
In rats famotidine appears to distribute only minimally into the CNS, and does not cross the placenta. It is not known whether the drug crosses the placenta in humans. Famotidine is distributed into milk in rats; however, it is not known whether the drug is distributed into milk in humans.
McEvoy G.K. (ed.). American Hospital Formulary Service-Drug Information 96. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1996 (Plus Supplements)., p. 2149
Famotidine is excreted principally in urine via glomerular filtration and tubular secretion. Approximately 25-30 or 65-80% of a dose is excreted unchanged in urine within 24 hours following oral or IV administration, respectively, and approximately 13-49 or 52-82% of a single 40 mg oral or IV dose respectively, is excreted within 72 hours. ... The remainder of an orally administered dose is eliminated in feces.
McEvoy G.K. (ed.). American Hospital Formulary Service-Drug Information 96. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1996 (Plus Supplements)., p. 2149
For more Absorption, Distribution and Excretion (Complete) data for FAMOTIDINE (7 total), please visit the HSDB record page.
Famotidine is metabolized in the liver to famotidine S-oxide (S-famotidine). The metabolite does not appear to inhibit gastric acid secretion. Orally administered famotidine undergoes minimal metabolism on first pass through the liver.
McEvoy G.K. (ed.). American Hospital Formulary Service-Drug Information 96. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1996 (Plus Supplements)., p. 2149
H2-receptor antagonists inhibit basal and nocturnal gastric acid secretion by competitive inhibition of the action of histamine at the histamine H2-receptors of the parietal cells. They also inhibit gastric acid secretion stimulated by food, betazole, pentagastrin, caffeine, insulin, and physiological vagal reflex. /Histamine H2-receptor antagonists/
USP Convention. USPDI - Drug Information for the Health Care Professional. 16th ed. Volume I. Rockville, MD: U.S. Pharmaceutical Convention, Inc. 1996 (Plus updates)., p. 1612
Weak inhibitor of hepatic cytochrome p450 mixed function oxidase system.
USP Convention. USPDI - Drug Information for the Health Care Professional. 16th ed. Volume I. Rockville, MD: U.S. Pharmaceutical Convention, Inc. 1996 (Plus updates)., p. 1612
Famotidine is a competitive H2 receptor antagonist that inhibits basal, overnight, and pentagastrin-stimulated gastric acid secretion. Pharmacologically, it is three times more potent than ranitidine and 20 times more potent than cimetidine.
American Medical Association, Council on Drugs. AMA Drug Evaluations Annual 1994. Chicago, IL: American Medical Association, 1994., p. 902
 LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
 DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.

GDUFA
DMF Review : Reviewed
Rev. Date : 2014-01-03
Pay. Date : 2013-11-25
DMF Number : 14309
Submission : 1999-07-22
Status : Active
Type : II

Certificate Number : R1-CEP 2009-161 - Rev 01
Issue Date : 2023-05-31
Type : Chemical
Substance Number : 1012
Status : Valid

Date of Issue : 2025-07-25
Valid Till : 2028-06-04
Written Confirmation Number : WC-0043
Address of the Firm :
 Gonane has API manufacturing expertise in new-age Corticosteroids, Hormones and other pharma raw materials.
Gonane has API manufacturing expertise in new-age Corticosteroids, Hormones and other pharma raw materials.
 Octavius Pharma has been empowering lives since 1980 by providing quality products like DC granules, APIs and FDFs.
Octavius Pharma has been empowering lives since 1980 by providing quality products like DC granules, APIs and FDFs.

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 11537
Submission : 1995-06-05
Status : Active
Type : II

Certificate Number : R1-CEP 1999-054 - Rev 03
Issue Date : 2022-02-18
Type : Chemical
Substance Number : 1012
Status : Valid

NDC Package Code : 45541-1175
Start Marketing Date : 2000-12-08
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

Registrant Name : Dong-A ST Co., Ltd.
Registration Date : 2018-02-08
Registration Number : 20180208-67-B-407-09
Manufacturer Name : Gedeon Richter Plc.
Manufacturer Address : Gyomroi ut 19-21., Budapest, 1103, Hungary

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
94
PharmaCompass offers a list of Famotidine API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Famotidine manufacturer or Famotidine supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Famotidine manufacturer or Famotidine supplier.
PharmaCompass also assists you with knowing the Famotidine API Price utilized in the formulation of products. Famotidine API Price is not always fixed or binding as the Famotidine Price is obtained through a variety of data sources. The Famotidine Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Famotidine manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Famotidine, including repackagers and relabelers. The FDA regulates Famotidine manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Famotidine API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Famotidine manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Famotidine supplier is an individual or a company that provides Famotidine active pharmaceutical ingredient (API) or Famotidine finished formulations upon request. The Famotidine suppliers may include Famotidine API manufacturers, exporters, distributors and traders.
click here to find a list of Famotidine suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Famotidine DMF (Drug Master File) is a document detailing the whole manufacturing process of Famotidine active pharmaceutical ingredient (API) in detail. Different forms of Famotidine DMFs exist exist since differing nations have different regulations, such as Famotidine USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Famotidine DMF submitted to regulatory agencies in the US is known as a USDMF. Famotidine USDMF includes data on Famotidine's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Famotidine USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Famotidine suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Famotidine Drug Master File in Japan (Famotidine JDMF) empowers Famotidine API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Famotidine JDMF during the approval evaluation for pharmaceutical products. At the time of Famotidine JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Famotidine suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Famotidine Drug Master File in Korea (Famotidine KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Famotidine. The MFDS reviews the Famotidine KDMF as part of the drug registration process and uses the information provided in the Famotidine KDMF to evaluate the safety and efficacy of the drug.
After submitting a Famotidine KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Famotidine API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Famotidine suppliers with KDMF on PharmaCompass.
A Famotidine CEP of the European Pharmacopoeia monograph is often referred to as a Famotidine Certificate of Suitability (COS). The purpose of a Famotidine CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Famotidine EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Famotidine to their clients by showing that a Famotidine CEP has been issued for it. The manufacturer submits a Famotidine CEP (COS) as part of the market authorization procedure, and it takes on the role of a Famotidine CEP holder for the record. Additionally, the data presented in the Famotidine CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Famotidine DMF.
A Famotidine CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Famotidine CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Famotidine suppliers with CEP (COS) on PharmaCompass.
A Famotidine written confirmation (Famotidine WC) is an official document issued by a regulatory agency to a Famotidine manufacturer, verifying that the manufacturing facility of a Famotidine active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Famotidine APIs or Famotidine finished pharmaceutical products to another nation, regulatory agencies frequently require a Famotidine WC (written confirmation) as part of the regulatory process.
click here to find a list of Famotidine suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Famotidine as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Famotidine API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Famotidine as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Famotidine and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Famotidine NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Famotidine suppliers with NDC on PharmaCompass.
Famotidine Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Famotidine GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Famotidine GMP manufacturer or Famotidine GMP API supplier for your needs.
A Famotidine CoA (Certificate of Analysis) is a formal document that attests to Famotidine's compliance with Famotidine specifications and serves as a tool for batch-level quality control.
Famotidine CoA mostly includes findings from lab analyses of a specific batch. For each Famotidine CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Famotidine may be tested according to a variety of international standards, such as European Pharmacopoeia (Famotidine EP), Famotidine JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Famotidine USP).
