



API Suppliers

US DMFs Filed

CEP/COS Certifications

JDMFs Filed
Other Certificates
Other Suppliers

USA (Orange Book)

Europe

Canada

Australia

South Africa
Uploaded Dossiers
U.S. Medicaid
Annual Reports
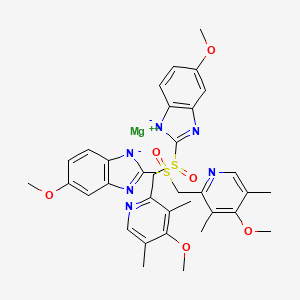

1. Esomeprazole
2. Esomeprazole Potassium
3. Esomeprazole Sodium
4. Esomeprazole Strontium
5. Esomeprazole Strontium Anhydrous
6. Nexium
7. Strontium, Esomeprazole
1. Omeprazole Magnesium
2. 161973-10-0
3. Prilosec Otc
4. H 168/68 Magnesium
5. 95382-33-5
6. Omeprazole (as Magnesium)
7. 426qfe7xlk
8. Magnesium;5-methoxy-2-[(4-methoxy-3,5-dimethylpyridin-2-yl)methylsulfinyl]benzimidazol-1-ide
9. Esomeprazole Magnesium Salt
10. Unii-426qfe7xlk
11. Omeprazole Magnesium [usan]
12. Omeprazole Magnesium [usan:usp]
13. Prilosec Otc (tn)
14. Esomeprazole(magnesium)
15. Omeprazole Magnesium Salt
16. Omeprazole Magnesium (usp)
17. Mls001165732
18. Schembl722792
19. Esomeprazole Magnesium (nexium)
20. Chembl1567328
21. Chebi:94401
22. H-168/68 Magnesium
23. Hms2878h13
24. Omeprazole Magnesium [vandf]
25. Mfcd06798050
26. Omeprazole Magnesium [mart.]
27. Omeprazole Magnesium [usp-rs]
28. Omeprazole Magnesium [who-dd]
29. Akos015896379
30. Akos025402081
31. Omeprazole Magnesium Salt [mi]
32. As-75082
33. Omeprazole Magnesium [orange Book]
34. Smr000550477
35. Omeprazole Magnesium [ep Monograph]
36. Omeprazole Magnesium [usp Impurity]
37. Omeprazole Magnesium [usp Monograph]
38. Ft-0657297
39. Sw220306-1
40. Talicia Component Omeprazole Magnesium
41. D05259
42. Omeprazole Magnesium Component Of Talicia
43. A810316
44. J-014249
45. Q-100195
46. Q27166253
47. 5-methoxy-1h-1,3-benzimidazol-2-yl (4-methoxy-3,5-dimethyl-2-pyridinyl)methyl Sulfoxide
48. (rs)-5-methoxy-2-(((4-methoxy-3,5-dimethyl-2-pyridinyl)methyl)sulfinyl)-1h-benzimidazole, Magnesium Salt (2:1)
49. 5-methoxy-2-(((4-methoxy-3,5-dimethyl-2-pyridinyl)methyl)sulfinyl)-1h-benzimidazole, Magnesium Salt
50. 5-methoxy-2-(((4-methoxy-3,5-dimethyl-2-pyridyl)methyl)sulfinyl)benzimidazole, Magnesium Salt (2:1)
51. Magnesium 5-methoxy-2-[(4-methoxy-3,5-dimethyl-2-pyridyl)methylsulfinyl]benzimidazol-1-ide;esomeprazole Magnesium(random Configuration)
52. Magnesium(2+) 5-methoxy-2-[(4-methoxy-3,5-dimethylpyridin-2-yl)methanesulfinyl]-1h-1,3-benzodiazol-1-ide 6-methoxy-2-[(4-methoxy-3,5-dimethylpyridin-2-yl)methanesulfinyl]-1h-1,3-benzodiazol-1-ide
53. Magnesium, Bis(6-methoxy-2-(((4-methoxy-3,5-dimethyl-2-pyridinyl)methyl)sulfinyl-.kappa.o)-1h-benzimidazolato-.kappa.n3)-, (t-4)-
| Molecular Weight | 713.1 g/mol |
|---|---|
| Molecular Formula | C34H36MgN6O6S2 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 14 |
| Rotatable Bond Count | 10 |
| Exact Mass | 712.1988169 g/mol |
| Monoisotopic Mass | 712.1988169 g/mol |
| Topological Polar Surface Area | 163 Ų |
| Heavy Atom Count | 49 |
| Formal Charge | 0 |
| Complexity | 453 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 2 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |
| 1 of 6 | |
|---|---|
| Drug Name | Esomeprazole magnesium |
| PubMed Health | Esomeprazole |
| Drug Classes | Gastric Acid Secretion Inhibitor, Gastrointestinal Agent |
| Drug Label | The active ingredient in NEXIUM (esomeprazole magnesium) Delayed-Release Capsules and NEXIUM (esomeprazole magnesium) For Delayed-Release Oral Suspension is bis(5-methoxy-2-[(S)-[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]sulfinyl]-1H-benzimidazole... |
| Active Ingredient | Esomeprazole magnesium |
| Dosage Form | Capsule, delayed release |
| Route | oral |
| Strength | 40mg; 20mg |
| Market Status | Tentative Approval |
| Company | Ranbaxy |
| 2 of 6 | |
|---|---|
| Drug Name | Nexium |
| Drug Label | The active ingredient in NEXIUM (esomeprazole magnesium) Delayed-Release Capsules and NEXIUM (esomeprazole magnesium) For Delayed-Release Oral Suspension is bis(5-methoxy-2-[(S)-[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]sulfinyl]-1H-benzimidazole... |
| Active Ingredient | Esomeprazole magnesium |
| Dosage Form | Capsule, delayed rel pellets; For suspension, delayed release |
| Route | Oral |
| Strength | eq 5mg base/packet; eq 20mg base/packet; eq 20mg base; eq 40mg base; eq 10mg base/packet; eq 40mg base/packet; eq 2.5mg base/packet |
| Market Status | Prescription |
| Company | Astrazeneca |
| 3 of 6 | |
|---|---|
| Drug Name | Omeprazole magnesium |
| Drug Label | The active ingredient in NEXIUM (esomeprazole magnesium) Delayed-Release Capsules and NEXIUM (esomeprazole magnesium) For Delayed-Release Oral Suspension is bis(5-methoxy-2-[(S)-[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]sulfinyl]-1H-benzimidazole... |
| Active Ingredient | Omeprazole magnesium |
| Dosage Form | Capsule, delayed release |
| Route | Oral |
| Strength | eq 20mg base |
| Market Status | Over the Counter |
| Company | Dr Reddys Labs |
| 4 of 6 | |
|---|---|
| Drug Name | Esomeprazole magnesium |
| PubMed Health | Esomeprazole |
| Drug Classes | Gastric Acid Secretion Inhibitor, Gastrointestinal Agent |
| Drug Label | The active ingredient in NEXIUM (esomeprazole magnesium) Delayed-Release Capsules and NEXIUM (esomeprazole magnesium) For Delayed-Release Oral Suspension is bis(5-methoxy-2-[(S)-[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]sulfinyl]-1H-benzimidazole... |
| Active Ingredient | Esomeprazole magnesium |
| Dosage Form | Capsule, delayed release |
| Route | oral |
| Strength | 40mg; 20mg |
| Market Status | Tentative Approval |
| Company | Ranbaxy |
| 5 of 6 | |
|---|---|
| Drug Name | Nexium |
| Drug Label | The active ingredient in NEXIUM (esomeprazole magnesium) Delayed-Release Capsules and NEXIUM (esomeprazole magnesium) For Delayed-Release Oral Suspension is bis(5-methoxy-2-[(S)-[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]sulfinyl]-1H-benzimidazole... |
| Active Ingredient | Esomeprazole magnesium |
| Dosage Form | Capsule, delayed rel pellets; For suspension, delayed release |
| Route | Oral |
| Strength | eq 5mg base/packet; eq 20mg base/packet; eq 20mg base; eq 40mg base; eq 10mg base/packet; eq 40mg base/packet; eq 2.5mg base/packet |
| Market Status | Prescription |
| Company | Astrazeneca |
| 6 of 6 | |
|---|---|
| Drug Name | Omeprazole magnesium |
| Drug Label | The active ingredient in NEXIUM (esomeprazole magnesium) Delayed-Release Capsules and NEXIUM (esomeprazole magnesium) For Delayed-Release Oral Suspension is bis(5-methoxy-2-[(S)-[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]sulfinyl]-1H-benzimidazole... |
| Active Ingredient | Omeprazole magnesium |
| Dosage Form | Capsule, delayed release |
| Route | Oral |
| Strength | eq 20mg base |
| Market Status | Over the Counter |
| Company | Dr Reddys Labs |
Anti-Ulcer Agents
Various agents with different action mechanisms used to treat or ameliorate PEPTIC ULCER or irritation of the gastrointestinal tract. This has included ANTIBIOTICS to treat HELICOBACTER INFECTIONS; HISTAMINE H2 ANTAGONISTS to reduce GASTRIC ACID secretion; and ANTACIDS for symptomatic relief. (See all compounds classified as Anti-Ulcer Agents.)
Proton Pump Inhibitors
Compounds that inhibit H(+)-K(+)-EXCHANGING ATPASE. They are used as ANTI-ULCER AGENTS and sometimes in place of HISTAMINE H2 ANTAGONISTS for GASTROESOPHAGEAL REFLUX. (See all compounds classified as Proton Pump Inhibitors.)








