
Synopsis
Synopsis
0
JDMF
0
KDMF
0
VMF
0
EDQM
0
USP
0
JP
0
Others
DRUG PRODUCT COMPOSITIONS
Regulatory FDF Prices
NA
0
Data Compilation #PharmaFlow
0
Weekly News Recap #Phispers
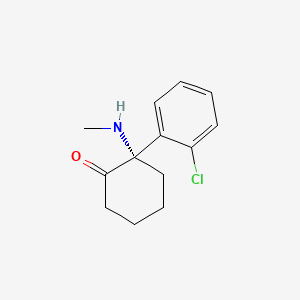

1. (-)-ketamine
2. (s)-2-(o-chlorophenyl)-2-(methylamino)cyclohexanone
3. Kataved
4. L-ketamine
5. S-ketamine
6. Spravato
1. (s)-ketamine
2. L-ketamine
3. (-)-ketamine
4. S-ketamine
5. 33643-46-8
6. (s)-(-)-ketamine
7. Spravato
8. S-(-)-ketamine
9. Ketaved
10. Keta-s
11. Ketamine, S-
12. Esketamine Free Base
13. Ketamine, (s)-
14. (s)-2-(o-chlorophenyl)-2-(methylamino)cyclohexanone
15. 50lfg02txd
16. Chebi:60799
17. Jnj-54135419
18. (+)-ketamine
19. (2s)-2-(2-chlorophenyl)-2-(methylamino)cyclohexanone
20. (2s)-2-(2-chlorophenyl)-2-(methylamino)cyclohexan-1-one
21. Cyclohexanone, 2-(2-chlorophenyl)-2-(methylamino)-, (2s)-
22. (2~{s})-2-(2-chlorophenyl)-2-(methylamino)cyclohexan-1-one
23. Cyclohexanone, 2-(2-chlorophenyl)-2-(methylamino)-, (s)-
24. Unii-50lfg02txd
25. Kataved
26. Esketamine [usan:inn:ban]
27. S-ketamin
28. Jc9
29. Esketamine [inn]
30. Esketamine (usan/inn)
31. Esketamine [usan]
32. Dsstox_cid_27787
33. Dsstox_rid_82562
34. Esketamine [who-dd]
35. Dsstox_gsid_47810
36. Chembl395091
37. Gtpl9152
38. Schembl5512024
39. Dtxsid6047810
40. 33643-46-8 (free Base)
41. Tox21_113206
42. Zinc35999642
43. Akos027321219
44. Db11823
45. Ncgc00185910-01
46. Cas-33643-46-8
47. D07283
48. Q2365493
49. (2s)-2-(2-chlorophenyl)-2-methylaminocyclohexan-1-one
50. (s)-2-(2-chlorophenyl)-2-(methylamino)cyclohexan-1-one
51. Cyclohexanone, 2-(2-chlorophenyl)-2-(methylamino)-, (2s)- (9ci)
52. Cyclohexanone, 2-(o-chlorophenyl)-2-(methylamino)-, (-)-
53. Cyclohexanone, 2-(o-chlorophenyl)-2-(methylamino)-, (-)- (8ci)
| Molecular Weight | 237.72 g/mol |
|---|---|
| Molecular Formula | C13H16ClNO |
| XLogP3 | 2.2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 2 |
| Exact Mass | 237.0920418 g/mol |
| Monoisotopic Mass | 237.0920418 g/mol |
| Topological Polar Surface Area | 29.1 Ų |
| Heavy Atom Count | 16 |
| Formal Charge | 0 |
| Complexity | 269 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
This drug is indicated in conjunction with an oral antidepressant for the treatment of treatment-resistant depression (TRD) in adults. Note: Esketamine is not approved as an anesthetic agent. The safety and effectiveness of esketamine as an anesthetic agent have not been established to this date.
FDA Label
Spravato, in combination with a SSRI or SNRI, is indicated for adults with treatment-resistant Major Depressive Disorder, who have not responded to at least two different treatments with antidepressants in the current moderate to severe depressive episode.
**General effects** Esketamine is considered a central nervous system (CNS) depressant agent. It may cause sedation, dizziness, and lethargy, among other symptoms. This drug has dissociative and antidepressant properties. Acutely, esketamine may impair attention, judgment, thinking, reaction speed, and motor skills. Two placebo-controlled studies were performed to evaluate the effects of ketamine on the ability to drive. The effects of esketamine 84 mg were comparable to placebo at 6 hours and 18 hours post ingestion. **Effects on cardiac electrophysiology** The effect of esketamine (84 mg nasal spray and 0.8 mg/kg esketamine intravenously infused over 40 minutes) on the QTc interval was studied in a randomized, double-blind, placebo-, and positive-controlled (moxifloxacin 400 mg), 4-period, crossover study in 60 healthy volunteers. A marked increase in heart rate (higher than 10 bpm) was measured in subjects receiving intranasal and intravenous esketamine. Summative evidence from both nonclinical and clinical data suggests a lack of clinically relevant QTc prolongation at the normal therapeutic dose of esketamine. **Effects on blood pressure** Eskestamine causes increases in systolic and/or diastolic blood pressure at all therapeutic doses. Peak blood pressure elevation after esketamine administration occurs about 40 minutes after administration and lasts approximately 4 hours. **Cognitive effects** In a study of healthy volunteers, one dose of this agent caused decline in cognitive performance 40 minutes after administration. Compared to subjects ingesting a placebo, esketamine-treated subjects required a higher level of effort to complete assigned cognitive tests at 40 minutes after administration. Cognitive performance and mental effort were found to be similar between esketamine and placebo at 2 hours after administration. Reports of long-term memory or cognitive impairment have been made following repeated ketamine misuse or abuse. No adverse effects of esketamine nasal spray on cognitive function were seen in a one-year open-label safety study. The long-term cognitive effects of esketamine have not been studied for more than a 1 year period, therefore, the risk of cognitive decline with long-term use is not yet confirmed.
Antidepressive Agents
Mood-stimulating drugs used primarily in the treatment of affective disorders and related conditions. Several MONOAMINE OXIDASE INHIBITORS are useful as antidepressants apparently as a long-term consequence of their modulation of catecholamine levels. The tricyclic compounds useful as antidepressive agents (ANTIDEPRESSIVE AGENTS, TRICYCLIC) also appear to act through brain catecholamine systems. A third group (ANTIDEPRESSIVE AGENTS, SECOND-GENERATION) is a diverse group of drugs including some that act specifically on serotonergic systems. (See all compounds classified as Antidepressive Agents.)
N06AX27
N - Nervous system
N01 - Anesthetics
N01A - Anesthetics, general
N01AX - Other general anesthetics
N01AX14 - Esketamine
N - Nervous system
N06 - Psychoanaleptics
N06A - Antidepressants
N06AX - Other antidepressants
N06AX27 - Esketamine
Absorption
Due to the fact that this drug is administered via nasal spray, absorption is rapid. The mean absolute bioavailability is approximately 48% after esketamine nasal spray administration. The time to achieve peak esketamine plasma concentration is 20 to 40 minutes after the last nasal spray of esketamine. Inter-subject variability of esketamine ranges from 27% to 66% for Cmax (maximum concentration) and 18% to 45% for AUC (area under the curve). The intra-subject variability of esketamine is about 15% for Cmax and 10% for AUC.
Route of Elimination
Less than 1% of a dose of nasal esketamine is measured as unchanged drug, excreted in the urine. Following intravenous (IV) or oral (PO) administration, esketamine-derived metabolites were mainly recovered in urine ( 78% of a radiolabeled dose), and a smaller percentage was measured in the feces ( 2% of a radiolabeled dose).
Volume of Distribution
The average steady-state volume of distribution of esketamine administered by the intravenous route is 709 L.
Clearance
The average clearance of esketamine is approximately 89 L/hour following intravenous administration. Elimination of the major esketamine metabolite, _noresketamine_, from plasma is slower than esketamine. The decrease of noresketamine plasma concentrations occurs in a biphasic fashion, with a more rapid decline for the first 4 hours post-administration, and an average terminal t1/2 of approximately 8 hours.
Esketamine is mainly metabolized to the _noresketamine_ metabolite by cytochrome P450 (CYP) enzymes, CYP2B6 and CYP3A4, and to a lesser extent, CYP2C9 and CYP2C19. Noresketamine is metabolized by cytochrome-dependent metabolic pathways followed by subsequent glucuronidation of metabolites.
The mean terminal half-life (t1/2) ranges from 7 to 12 hours.
Esketamine, the S-enantiomer of racemic ketamine, is a non-selective, non-competitive antagonist of the N-methyl-D-aspartate (NMDA) receptor, an ionotropic glutamate receptor. The exact mechanism by which esketamine acts as an antidepressant is unknown. The primary circulating metabolite of esketamine (_noresketamine_) shows activity at the same receptor with a weaker affinity.
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
ABOUT THIS PAGE
80
PharmaCompass offers a list of Esketamine Hydrochloride API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Esketamine Hydrochloride manufacturer or Esketamine Hydrochloride supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Esketamine Hydrochloride manufacturer or Esketamine Hydrochloride supplier.
PharmaCompass also assists you with knowing the Esketamine Hydrochloride API Price utilized in the formulation of products. Esketamine Hydrochloride API Price is not always fixed or binding as the Esketamine Hydrochloride Price is obtained through a variety of data sources. The Esketamine Hydrochloride Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Esketamine Hydrochloride manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Esketamine Hydrochloride, including repackagers and relabelers. The FDA regulates Esketamine Hydrochloride manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Esketamine Hydrochloride API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Esketamine Hydrochloride manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Esketamine Hydrochloride supplier is an individual or a company that provides Esketamine Hydrochloride active pharmaceutical ingredient (API) or Esketamine Hydrochloride finished formulations upon request. The Esketamine Hydrochloride suppliers may include Esketamine Hydrochloride API manufacturers, exporters, distributors and traders.
click here to find a list of Esketamine Hydrochloride suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Esketamine Hydrochloride DMF (Drug Master File) is a document detailing the whole manufacturing process of Esketamine Hydrochloride active pharmaceutical ingredient (API) in detail. Different forms of Esketamine Hydrochloride DMFs exist exist since differing nations have different regulations, such as Esketamine Hydrochloride USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Esketamine Hydrochloride DMF submitted to regulatory agencies in the US is known as a USDMF. Esketamine Hydrochloride USDMF includes data on Esketamine Hydrochloride's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Esketamine Hydrochloride USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Esketamine Hydrochloride suppliers with USDMF on PharmaCompass.
A Esketamine Hydrochloride CEP of the European Pharmacopoeia monograph is often referred to as a Esketamine Hydrochloride Certificate of Suitability (COS). The purpose of a Esketamine Hydrochloride CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Esketamine Hydrochloride EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Esketamine Hydrochloride to their clients by showing that a Esketamine Hydrochloride CEP has been issued for it. The manufacturer submits a Esketamine Hydrochloride CEP (COS) as part of the market authorization procedure, and it takes on the role of a Esketamine Hydrochloride CEP holder for the record. Additionally, the data presented in the Esketamine Hydrochloride CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Esketamine Hydrochloride DMF.
A Esketamine Hydrochloride CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Esketamine Hydrochloride CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Esketamine Hydrochloride suppliers with CEP (COS) on PharmaCompass.
A Esketamine Hydrochloride written confirmation (Esketamine Hydrochloride WC) is an official document issued by a regulatory agency to a Esketamine Hydrochloride manufacturer, verifying that the manufacturing facility of a Esketamine Hydrochloride active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Esketamine Hydrochloride APIs or Esketamine Hydrochloride finished pharmaceutical products to another nation, regulatory agencies frequently require a Esketamine Hydrochloride WC (written confirmation) as part of the regulatory process.
click here to find a list of Esketamine Hydrochloride suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Esketamine Hydrochloride as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Esketamine Hydrochloride API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Esketamine Hydrochloride as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Esketamine Hydrochloride and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Esketamine Hydrochloride NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Esketamine Hydrochloride suppliers with NDC on PharmaCompass.
Esketamine Hydrochloride Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Esketamine Hydrochloride GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Esketamine Hydrochloride GMP manufacturer or Esketamine Hydrochloride GMP API supplier for your needs.
A Esketamine Hydrochloride CoA (Certificate of Analysis) is a formal document that attests to Esketamine Hydrochloride's compliance with Esketamine Hydrochloride specifications and serves as a tool for batch-level quality control.
Esketamine Hydrochloride CoA mostly includes findings from lab analyses of a specific batch. For each Esketamine Hydrochloride CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Esketamine Hydrochloride may be tested according to a variety of international standards, such as European Pharmacopoeia (Esketamine Hydrochloride EP), Esketamine Hydrochloride JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Esketamine Hydrochloride USP).

