



API Suppliers

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
0
Listed Suppliers
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
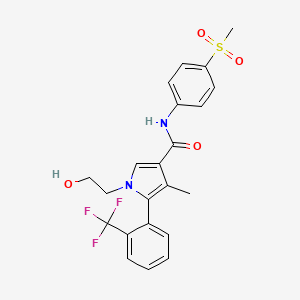

1. 1-(2-hydroxyethyl)-4-methyl-n-(4-(methylsulfonyl)phenyl)-5-(2-(trifluoromethyl)phenyl)-1h-pyrrole-3-carboxamide
2. Cs-3150
1. 1632006-28-0
2. Cs-3150
3. Esaxerenone [inn]
4. Xl-550
5. N62tgj04a1
6. 1-(2-hydroxyethyl)-4-methyl-n-(4-methylsulfonylphenyl)-5-[2-(trifluoromethyl)phenyl]pyrrole-3-carboxamide
7. Unii-n62tgj04a1
8. 1-(2-hydroxyethyl)-4-methyl-n-(4-(methylsulfonyl)phenyl)-5-(2-(trifluoromethyl)phenyl)-1h-pyrrole-3-carboxamide
9. Chembl2181932
10. 1h-pyrrole-3-carboxamide, 1-(2-hydroxyethyl)-4-methyl-n-(4-(methylsulfonyl)phenyl)-5-(2-(trifluoromethyl)phenyl)-, (5s)-
11. E6r
12. Esaxerenone (jan/inn)
13. Esaxerenone [jan]
14. Esaxerenone [who-dd]
15. Gtpl9894
16. Schembl1381714
17. Dtxsid601336896
18. Glxc-26595
19. Ex-a2595
20. Bdbm50398059
21. Cs-3150 (esaxerenone;xl-550)
22. Akos032946523
23. Cs-8044
24. Db15207
25. Cs-3150; Xl-550
26. Ac-36169
27. Ms-28595
28. Hy-100471
29. A16925
30. D10892
31. Q27284603
32. (+/-)-1-(2-hydroxyethyl)-4-methyl-n-[4-(methylsulfonyl)phenyl]-5-[2-(trifluoromethyl)phenyl]-1h-pyrrole-3-carboxamide
33. 1-(2-hydroxyethyl)-n-(4-methanesulfonylphenyl)-4-methyl-5-[2-(trifluoromethyl)phenyl]-1h-pyrrole-3-carboxamide
34. 880780-76-7
| Molecular Weight | 466.5 g/mol |
|---|---|
| Molecular Formula | C22H21F3N2O4S |
| XLogP3 | 2.9 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 6 |
| Exact Mass | g/mol |
| Monoisotopic Mass | g/mol |
| Topological Polar Surface Area | 96.8 |
| Heavy Atom Count | 32 |
| Formal Charge | 0 |
| Complexity | 747 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |


