
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
KDMF
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
South Africa
0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
Regulatory FDF Prices
NA
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Braftovi
2. Lgx818
1. Lgx818
2. 1269440-17-6
3. Lgx-818
4. Braftovi
5. Encorafenib (lgx818)
6. Nvp-lgx818-nxa
7. Nvp-lgx-818-nxa
8. Nvp-lgx818
9. 8l7891mrb6
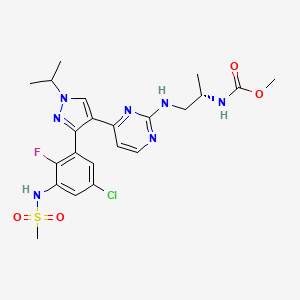
10. Methyl N-[(2s)-1-[[4-[3-[5-chloro-2-fluoro-3-(methanesulfonamido)phenyl]-1-propan-2-ylpyrazol-4-yl]pyrimidin-2-yl]amino]propan-2-yl]carbamate
11. Carbamic Acid, N-[(1s)-2-[[4-[3-[5-chloro-2-fluoro-3-[(methylsulfonyl)amino]phenyl]-1-(1-methylethyl)-1h-pyrazol-4-yl]-2-pyrimidinyl]amino]-1-methylethyl]-, Methyl Ester
12. Encorafenib [usan:inn]
13. Unii-8l7891mrb6
14. Lgx 818
15. Braftovi (tn)
16. Carbamic Acid, N-((1s)-2-((4-(3-(5-chloro-2-fluoro-3-((methylsulfonyl)amino)phenyl)-1-(1-methylethyl)-1h-pyrazol-4-yl)-2-pyrimidinyl)amino)-1-methylethyl)-, Methyl Ester
17. Encorafenib [mi]
18. Encorafenib(lgx-818)
19. Lgx-818(encorafenib)
20. Encorafenib [inn]
21. Encorafenib [jan]
22. Encorafenib [usan]
23. Encorafenib [who-dd]
24. Encorafenib (jan/usan/inn)
25. Gtpl7908
26. Schembl8228295
27. Chembl3301612
28. Dtxsid00155347
29. Encorafenib [orange Book]
30. Bdbm221688
31. Bcp08458
32. Ex-a1587
33. Mfcd25976758
34. Nsc778304
35. Nsc800093
36. S7108
37. Zinc68249103
38. Ccg-269960
39. Db11718
40. Nsc-778304
41. Nsc-800093
42. Ncgc00378599-03
43. Ac-30230
44. As-35201
45. Hy-15605
46. A13226
47. D11053
48. Us9314464, 9
49. Q15409405
50. (s)-methyl (1-((4-(3-(5-chloro-2-fluoro-3-(methylsulfonamido)phenyl)-1-isopropyl-1h-pyrazol-4-yl)pyrimidin-2-yl)amino)propan-2-yl)carbamate
51. Methyl N-((2s)-1-((4-(3-(5-chloro-2-fluoro-3-(methanesulfonamido)phenyl)(-1-(propan-2-yl)-1h-pyrazol-4-yl(pyrimidin-2-yl)amino)propan-2-yl)carbamate
52. Methyl N-[(2s)-1-({4-[3-(5-chloro-2-fluoro-3-methanesulfonamidophenyl)-1-(propan-2-yl)-1h-pyrazol-4-yl]pyrimidin-2-yl}amino)propan-2-yl]carbamate
| Molecular Weight | 540.0 g/mol |
|---|---|
| Molecular Formula | C22H27ClFN7O4S |
| XLogP3 | 2.7 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 10 |
| Exact Mass | 539.1517794 g/mol |
| Monoisotopic Mass | 539.1517794 g/mol |
| Topological Polar Surface Area | 149 Ų |
| Heavy Atom Count | 36 |
| Formal Charge | 0 |
| Complexity | 836 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Used in combination with [Binimetinib] in metastatic melanoma with a BRAF V600E or V600K mutation, as detected by an FDA-approved test.
Encorafenib is indicated:
- in combination with binimetinib is indicated for the treatment of adult patients with unresectable or metastatic melanoma with a BRAF V600 mutation
- in combination with cetuximab, for the treatment of adult patients with metastatic colorectal cancer (CRC) with a BRAF V600E mutation, who have received prior systemic therapy
Treatment of colorectal carcinoma
Treatment of melanoma
Encorafenib has shown improved efficacy in the treatment of metastatic melanoma. Encorafenib, a selective BRAF inhibitor (BRAFi), has a pharmacologic profile that is distinct from that of other clinically active BRAFis. Once-daily dosing of single-agent encorafenib has a distinct tolerability profile and shows varying antitumor activity across BRAFi-pretreated and BRAFi-nave patients with advanced/metastatic stage melanoma.
L01EC03
L - Antineoplastic and immunomodulating agents
L01 - Antineoplastic agents
L01E - Protein kinase inhibitors
L01EC - B-raf serine-threonine kinase (braf) inhibitors
L01EC03 - Encorafenib
Absorption
After oral administration, the median Tmax of encorafenib is 2 hours. At least 86% of the dose is absorbed. Administration of a single dose of BRAFTOVI 100 mg (0.2 times the recommended dose) with a high-fat, high-calorie meal (comprised of approximately 150 calories from protein, 350 calories from carbohydrates, and 500 calories from fat) decreased the mean maximum encorafenib concentration (Cmax) by 36% with no effect on AUC (area under the curve).
Route of Elimination
Following a single oral dose of 100 mg radiolabeled encorafenib, 47% (5% unchanged) of the administered dose was recovered in the feces and 47% (2% unchanged) was recovered in the urine.
Volume of Distribution
The blood-to-plasma concentration ratio is 0.58. The geometric mean (CV%) of apparent volume of distribution is 164 L (70%).
Clearance
The apparent clearance is 14 L/h (54%) at day 1, increasing to 32 L/h (59%) at steady-state.
The primary metabolic pathway is N-dealkylation, with CYP3A4 as the main contributor (83%) to total oxidative clearance of encorafenib in human liver microsomes, followed by CYP2C19 (16%) and CYP2D6 (1%).
The mean (CV%) terminal half-life (t1/2) of encorafenib is 3.5 hours (17%)
Encorafenib is a kinase inhibitor that specifically targets BRAF V600E, as well as wild-type BRAF and CRAF while tested with in vitro cell-free assays with IC50 values of 0.35, 0.47, and 0.3 nM, respectively. Mutations in the BRAF gene, including BRAF V600E, result in activated BRAF kinases that mahy stimulate tumor cell growth. Encorafenib is able to bind to other kinases in vitro including JNK1, JNK2, JNK3, LIMK1, LIMK2, MEK4, and STK36 and significantly reduce ligand binding to these kinases at clinically achievable concentrations ( 0.9 M). In efficacy studies, encorafenib inhibited the in vitro cell growth of tumor cell lines that express BRAF V600 E, D, and K mutations. In mice implanted with tumor cells expressing the BRAF V600E mutation, encorafenib induced tumor regressions associated with RAF/MEK/ERK pathway suppression. Encorafenib and binimetinib target two different kinases in the RAS/RAF/MEK/ERK pathway. Compared with either drug alone, co-administration of encorafenib and binimetinib result in greater anti-proliferative activity in vitro in BRAF mutation-positive cell lines and greater anti-tumor activity with respect to tumor growth inhibition in BRAF V600E mutant human melanoma xenograft studies in mice. In addition to the above, the combination of encorafenib and binimetinib acted to delay the emergence of resistance in BRAF V600E mutant human melanoma xenografts in mice compared with the administration of either drug alone.

NDC Package Code : 62009-1913
Start Marketing Date : 2018-09-28
End Marketing Date : 2026-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT
USDMF, CEP/COS, JDMF, EU-WC, NDC, KDMF, VMF, Others
USDMF, CEP/COS, JDMF, EU-WC, NDC, KDMF, VMF, Others

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 41902
Submission : 2025-06-13
Status : Active
Type : II

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF

GDUFA
DMF Review : Reviewed
Rev. Date : 2022-01-04
Pay. Date : 2021-12-20
DMF Number : 36630
Submission : 2021-12-21
Status : Active
Type : II

NDC Package Code : 59651-641
Start Marketing Date : 2023-12-18
End Marketing Date : 2026-12-31
Dosage Form (Strength) : POWDER (50kg/50kg)
Marketing Category : BULK INGREDIENT



GDUFA
DMF Review : Reviewed
Rev. Date : 2022-04-06
Pay. Date : 2022-01-07
DMF Number : 35756
Submission : 2021-03-31
Status : Active
Type : II

Date of Issue : 2025-09-19
Valid Till : 2028-05-05
Written Confirmation Number : WC-0349
Address of the Firm :

NDC Package Code : 54893-0114
Start Marketing Date : 2021-03-30
End Marketing Date : 2027-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT




USDMF, CEP/COS, JDMF, EU-WC, NDC, KDMF, VMF, Others
USDMF, CEP/COS, JDMF, EU-WC, NDC, KDMF, VMF, Others

NDC Package Code : 81955-0026
Start Marketing Date : 2018-06-27
End Marketing Date : 2026-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF


 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
GDUFA
DMF Review : Complete
Rev. Date : 2022-01-04
Pay. Date : 2021-12-20
DMF Number : 36630
Submission : 2021-12-21
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2022-04-06
Pay. Date : 2022-01-07
DMF Number : 35756
Submission : 2021-03-31
Status : Active
Type : II

USDMF
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 41902
Submission : 2025-06-13
Status : Active
Type : II

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Date of Issue : 2025-09-19
Valid Till : 2028-05-05
Written Confirmation Number : WC-0349
Address of the Firm : Unit-II, Sy. Nos. 50, 53, 53/A, 54 & 54/A,Kardanur (Village), Patancheru (Mandal...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]About the Company : Founded in 1986 by Mr. P.V. Ramaprasad Reddy, Mr. K. Nityananda Reddy and a small group of highly committed professionals, Aurobindo Pharma was born off a vision. The company comme...

About the Company : Hy-Gro Chemicals Pharmtek Pvt. Ltd. (Hy-Gro) is a fast growing pharmaceutical company engaged in the manufacture of Active Pharmaceutical Ingredients (APIs), Advanced Intermediates...

About the Company : ICROM is specialized in the manufacturing and contract manufacturing of Active Pharmaceutical Ingredients (APIs) and GMP intermediates. ICROM is the partner of choice for pharmaceu...

About the Company : Sichuan Taienkang Pharmaceutical Co., Ltd., established in 2020, is a subsidiary of Guangdong Taienkang Pharmaceutical Co., Ltd. The company specializes in the R&D, production, and...

About the Company : Viyash, true to its name, literally represents honesty and leadership in every sense. We are an integrated pharmaceutical company with a strong portfolio of niche formulations, ...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
N,N-Dimethylformamide Dimethyl Acetal (DMF-DMA)
CAS Number : 4637-24-5
End Use API : Encorafenib
About The Company : Darshan Pharmachem, founded in 2011 in Ankleshwar, is a leading pharmaceutical company specializing in high-quality and innovative API intermediates. We are com...

1-(3-iodo-1-isopropyl-1H-pyrazol-4-yl)ethanone
CAS Number : 1269440-49-4
End Use API : Encorafenib
About The Company : Ocimum Labs was founded in 2018 and started its R&D activities with a cutting-edge facility. The Hyderabad-based firm focuses on process research and developmen...

N,N-Dimethylformamide dimethyl acetal
CAS Number : 4637-24-5
End Use API : Encorafenib
About The Company : Shree Ganesh Remedies is established Since 1995 with an aim to manufacture, supply and export best quality of Pharmaceutical-intermediates, Bulk drugs and Speci...

N,N-Dimethylformamide Dimethyl Acetal
CAS Number : 4637-24-5
End Use API : Encorafenib
About The Company : SLN Pharmachem was setup in 1997 with an objective to provide technology based products (Intermediates) for Pharmaceutical, Cosmetic and Fine Chemical Industry....

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Global Sales Information

Market Place

Patents & EXCLUSIVITIES
ABOUT THIS PAGE
63
PharmaCompass offers a list of Encorafenib API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Encorafenib manufacturer or Encorafenib supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Encorafenib manufacturer or Encorafenib supplier.
PharmaCompass also assists you with knowing the Encorafenib API Price utilized in the formulation of products. Encorafenib API Price is not always fixed or binding as the Encorafenib Price is obtained through a variety of data sources. The Encorafenib Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Encorafenib manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Encorafenib, including repackagers and relabelers. The FDA regulates Encorafenib manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Encorafenib API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Encorafenib manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Encorafenib supplier is an individual or a company that provides Encorafenib active pharmaceutical ingredient (API) or Encorafenib finished formulations upon request. The Encorafenib suppliers may include Encorafenib API manufacturers, exporters, distributors and traders.
click here to find a list of Encorafenib suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Encorafenib DMF (Drug Master File) is a document detailing the whole manufacturing process of Encorafenib active pharmaceutical ingredient (API) in detail. Different forms of Encorafenib DMFs exist exist since differing nations have different regulations, such as Encorafenib USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Encorafenib DMF submitted to regulatory agencies in the US is known as a USDMF. Encorafenib USDMF includes data on Encorafenib's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Encorafenib USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Encorafenib suppliers with USDMF on PharmaCompass.
A Encorafenib written confirmation (Encorafenib WC) is an official document issued by a regulatory agency to a Encorafenib manufacturer, verifying that the manufacturing facility of a Encorafenib active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Encorafenib APIs or Encorafenib finished pharmaceutical products to another nation, regulatory agencies frequently require a Encorafenib WC (written confirmation) as part of the regulatory process.
click here to find a list of Encorafenib suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Encorafenib as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Encorafenib API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Encorafenib as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Encorafenib and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Encorafenib NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Encorafenib suppliers with NDC on PharmaCompass.
Encorafenib Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Encorafenib GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Encorafenib GMP manufacturer or Encorafenib GMP API supplier for your needs.
A Encorafenib CoA (Certificate of Analysis) is a formal document that attests to Encorafenib's compliance with Encorafenib specifications and serves as a tool for batch-level quality control.
Encorafenib CoA mostly includes findings from lab analyses of a specific batch. For each Encorafenib CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Encorafenib may be tested according to a variety of international standards, such as European Pharmacopoeia (Encorafenib EP), Encorafenib JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Encorafenib USP).

