
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Europe
0
Australia
0
South Africa
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
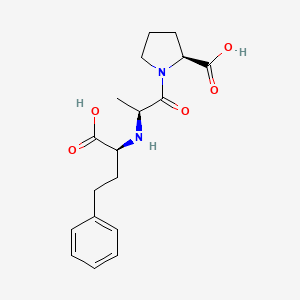

1. 1-(n-((s)-1-carboxy-3-phenylpropyl)-l-alanyl)-l-proline Dihydrate
2. Enalaprilat Anhydrous
3. Enalaprilat Citrate, Anhydrous
4. Enalaprilat Dihydrate
5. Enalaprilat, (r)-isomer, Anhydrous
6. Enalaprilic Acid
7. Mk 422
8. Mk-422
9. Mk422
10. Pres Iv
11. Vasotec
12. Xanef
1. 76420-72-9
2. Enalapril Acid
3. Enalaprilate
4. Enalaprilatum
5. Enalaprilic Acid
6. Enalaprilat Anhydrous
7. Enalapril Diacid
8. Enalaprilat (anhydrous)
9. (s)-1-((s)-2-(((s)-1-carboxy-3-phenylpropyl)amino)propanoyl)pyrrolidine-2-carboxylic Acid
10. N-[(1s)-1-carboxy-3-phenylpropyl]-l-alanyl-l-proline
11. Mk-422
12. Enalaprilat [inn]
13. Chebi:4786
14. Q508q118jm
15. 1-((2s)-2-{[(1s)-1-carboxy-3-phenylpropyl]amino}propanoyl)-l-proline
16. ((s)-1-carboxy-3-phenylpropyl)-l-alanyl-l-proline
17. Eal
18. L-proline, N-((1s)-1-carboxy-3-phenylpropyl)-l-alanyl-
19. L-proline, N-[(1s)-1-carboxy-3-phenylpropyl]-l-alanyl-
20. Enalaprilat Inhibitor
21. (2s)-1-[(2s)-2-[[(1s)-1-carboxy-3-phenylpropyl]amino]propanoyl]pyrrolidine-2-carboxylic Acid
22. Smr000466359
23. Enalaprilat [spanish]
24. Mk 421 Diacid
25. Chembl577
26. Enalaprilate [french]
27. Enalaprilatum [latin]
28. Mk 422
29. Unii-q508q118jm
30. Enalaprilat,(s)
31. (s)-1-((s)-2-((s)-1-carboxy-3-phenylpropylamino)propanoyl)pyrrolidine-2-carboxylic Acid
32. (2s)-1-((2s)-2-(((1s)-1-carboxy-3-phenylpropyl)amino)propanoyl)pyrrolidine-2-carboxylic Acid
33. (2s)-1-[(2s)-2-{[(1s)-1-carboxy-3-phenylpropyl]amino}propanoyl]pyrrolidine-2-carboxylic Acid
34. Einecs 278-459-3
35. Mfcd00865786
36. Schembl37289
37. (2s)-1-[(2s)-2-[[(2s)-1-hydroxy-1-oxo-4-phenylbutan-2-yl]amino]propanoyl]pyrrolidine-2-carboxylic Acid
38. Mls000759476
39. Mls001424138
40. Bidd:gt0752
41. Gtpl6332
42. N-(1(s)-carboxy-3-phenylpropyl)-l-alanyl-l-proline
43. Enalaprilat, Analytical Standard
44. Dtxsid0048975
45. Hy-b0231a
46. Hms2051h16
47. Hms2089p04
48. Hms2232b19
49. Hms3263o13
50. Pharmakon1600-01503833
51. Zinc3812851
52. Tox21_501036
53. Bdbm50367254
54. Nsc760053
55. Akos015840130
56. Akos015892570
57. Ccg-101042
58. Db09477
59. Lp01036
60. Nc00292
61. Sdccgsbi-0633781.p001
62. Ncgc00164593-01
63. Ncgc00164593-11
64. Ncgc00261721-01
65. As-13013
66. Cs-0012359
67. E1302
68. Ab00698268-05
69. Ab00698268-07
70. Ab00698268-08
71. Ab00698268_09
72. Ab00698268_10
73. Enalapril Diacid Dihydrate Anhydrous [mi]
74. 420e729
75. Enalapril Maleate Impurity C [ep Impurity]
76. Sr-01000763426
77. Q5375179
78. Sr-01000763426-3
79. W-104365
80. N-[(s)-1-carboxy-3-phenyl-propyl]-l-alanyl-l-proline
81. 1-(n-((s)-1-carboxy-3-phenylpropyl)-l-alanyl)-l-proline
82. L-proline, 1-(n-(1-carboxt-3-phenylpropyl)-l-alanyl)-, (s)-
83. L-proline, 1-(n-(1-carboxy-3-phenylpropyl)-l-alanyl)-, (s)-
84. (s)-1-((s)-2-(((s)-1-carboxy-3-phenylpropyl)amino)propanoyl)pyrrolidine-2-carboxylicacid
85. (2s)-1-[(2s)-2-[[(1s)-1-carboxy-3-phenylpropyl]amino]propanoyl]pyrrolidine-2-carboxylic Acid (enalaprilat)
| Molecular Weight | 348.4 g/mol |
|---|---|
| Molecular Formula | C18H24N2O5 |
| XLogP3 | -0.7 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 8 |
| Exact Mass | 348.16852187 g/mol |
| Monoisotopic Mass | 348.16852187 g/mol |
| Topological Polar Surface Area | 107 Ų |
| Heavy Atom Count | 25 |
| Formal Charge | 0 |
| Complexity | 490 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Enalaprilat |
| PubMed Health | Enalaprilat (Injection) |
| Drug Classes | Antihypertensive, Cardiovascular Agent, Renal Protective Agent |
| Drug Label | Enalaprilat injection USP is a sterile aqueous solution for intravenous administration. Enalaprilat is an angiotensin-converting enzyme inhibitor. It is chemically described as (S)-1-[N-(1-carboxy-3-phenylpropyl)-L-alanyl]-L-proline dihydrate. Its st... |
| Active Ingredient | Enalaprilat |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 1.25mg/ml |
| Market Status | Prescription |
| Company | Bedford; Hospira; Hikma Farmaceutica; Teva Pharms Usa |
| 2 of 2 | |
|---|---|
| Drug Name | Enalaprilat |
| PubMed Health | Enalaprilat (Injection) |
| Drug Classes | Antihypertensive, Cardiovascular Agent, Renal Protective Agent |
| Drug Label | Enalaprilat injection USP is a sterile aqueous solution for intravenous administration. Enalaprilat is an angiotensin-converting enzyme inhibitor. It is chemically described as (S)-1-[N-(1-carboxy-3-phenylpropyl)-L-alanyl]-L-proline dihydrate. Its st... |
| Active Ingredient | Enalaprilat |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 1.25mg/ml |
| Market Status | Prescription |
| Company | Bedford; Hospira; Hikma Farmaceutica; Teva Pharms Usa |
Enalaprilat injection is indicated for the treatment of hypertension when oral therapy is not practical.
FDA Label
Enalaprilat injection results in the reduction of both supine and standing systolic and diastolic blood pressure, usually with no orthostatic component. Symptomatic postural hypotension is therefore infrequent, although it might be anticipated in volume-depleted patients. The onset of action usually occurs within fifteen minutes of administration with the maximum effect occurring within one to four hours. The abrupt withdrawal of enalaprilat has not been associated with a rapid increase in blood pressure. The duration of hemodynamic effects appears to be dose-related. However, for the recommended dose, the duration of action in most patients is approximately six hours. Following administration of enalapril, there is an increase in renal blood flow; glomerular filtration rate is usually unchanged. The effects appear to be similar in patients with renovascular hypertension.
Angiotensin-Converting Enzyme Inhibitors
A class of drugs whose main indications are the treatment of hypertension and heart failure. They exert their hemodynamic effect mainly by inhibiting the renin-angiotensin system. They also modulate sympathetic nervous system activity and increase prostaglandin synthesis. They cause mainly vasodilation and mild natriuresis without affecting heart rate and contractility. (See all compounds classified as Angiotensin-Converting Enzyme Inhibitors.)
Antihypertensive Agents
Drugs used in the treatment of acute or chronic vascular HYPERTENSION regardless of pharmacological mechanism. Among the antihypertensive agents are DIURETICS; (especially DIURETICS, THIAZIDE); ADRENERGIC BETA-ANTAGONISTS; ADRENERGIC ALPHA-ANTAGONISTS; ANGIOTENSIN-CONVERTING ENZYME INHIBITORS; CALCIUM CHANNEL BLOCKERS; GANGLIONIC BLOCKERS; and VASODILATOR AGENTS. (See all compounds classified as Antihypertensive Agents.)
Absorption
Enalaprilat is poorly absorbed following oral administration, and is therefore only available as an intravenous injection.
Route of Elimination
Excretion of enalaprilat is primarily renal with more than 90 percent of an administered dose recovered in the urine as unchanged drug within 24 hours.
Clearance
The disposition of enalaprilat in patients with renal insufficiency is similar to that in patients with normal renal function until the glomerular filtration rate is 30 mL/min or less. Renal clearance was 158 47 ml/min.
Both enalapril and enalaprilat undergo renal excretion without further metabolism.
11 hr
Enalaprilat is the active metabolite of the orally available pro-drug, enalapril. Used in the treatment of hypertension, enalapril is an ACE inhibitor that prevents Angiotensin Converting Enzyme (ACE) from transforming angiotensin I into angiotensin II. As angiotensin II is responsible for vasoconstriction and sodium reabsorption in the proximal tubule of the kidney, down-regulation of this protein results in reduced blood pressure and blood fluid volume
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
19
PharmaCompass offers a list of Enalaprilat API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Enalaprilat manufacturer or Enalaprilat supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Enalaprilat manufacturer or Enalaprilat supplier.
PharmaCompass also assists you with knowing the Enalaprilat API Price utilized in the formulation of products. Enalaprilat API Price is not always fixed or binding as the Enalaprilat Price is obtained through a variety of data sources. The Enalaprilat Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Enalaprilat manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Enalaprilat, including repackagers and relabelers. The FDA regulates Enalaprilat manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Enalaprilat API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Enalaprilat manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Enalaprilat supplier is an individual or a company that provides Enalaprilat active pharmaceutical ingredient (API) or Enalaprilat finished formulations upon request. The Enalaprilat suppliers may include Enalaprilat API manufacturers, exporters, distributors and traders.
click here to find a list of Enalaprilat suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Enalaprilat DMF (Drug Master File) is a document detailing the whole manufacturing process of Enalaprilat active pharmaceutical ingredient (API) in detail. Different forms of Enalaprilat DMFs exist exist since differing nations have different regulations, such as Enalaprilat USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Enalaprilat DMF submitted to regulatory agencies in the US is known as a USDMF. Enalaprilat USDMF includes data on Enalaprilat's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Enalaprilat USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Enalaprilat suppliers with USDMF on PharmaCompass.
Enalaprilat Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Enalaprilat GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Enalaprilat GMP manufacturer or Enalaprilat GMP API supplier for your needs.
A Enalaprilat CoA (Certificate of Analysis) is a formal document that attests to Enalaprilat's compliance with Enalaprilat specifications and serves as a tool for batch-level quality control.
Enalaprilat CoA mostly includes findings from lab analyses of a specific batch. For each Enalaprilat CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Enalaprilat may be tested according to a variety of international standards, such as European Pharmacopoeia (Enalaprilat EP), Enalaprilat JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Enalaprilat USP).

