
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
South Africa
0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
US Medicaid
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
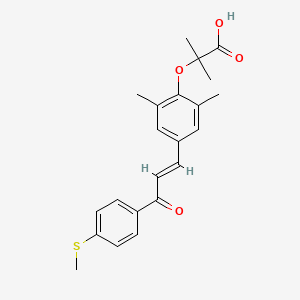

1. 2-(2,6-dimethyl-4-(3-(4-(methylthio)phenyl)-3-oxo-1-propenyl)phenoxyl)-2-methylpropanoic Acid
2. Gft505
1. 923978-27-2
2. Gft505
3. Gft-505
4. 824932-88-9
5. Elafibranor [inn]
6. Elafibranor [usan]
7. Gft 505
8. Elafibranor(gft505)
9. 2-[2,6-dimethyl-4-[(e)-3-(4-methylsulfanylphenyl)-3-oxoprop-1-enyl]phenoxy]-2-methylpropanoic Acid
10. 2j3h5c81a5
11. Elafibranor (usan)
12. (e)-2-(2,6-dimethyl-4-(3-(4-(methylthio)phenyl)-3-oxoprop-1-en-1-yl)phenoxy)-2-methylpropanoic Acid
13. 2-(2,6-dimethyl-4-(3-(4-(methylthio)phenyl)-3-oxoprop-1-en-1-yl)phenoxy)-2-methylpropanoic Acid
14. 2-{2,6-dimethyl-4-[(1e)-3-[4-(methylsulfanyl)phenyl]-3-oxoprop-1-en-1-yl]phenoxy}-2-methylpropanoic Acid
15. Propanoic Acid, 2-(2,6-dimethyl-4-(3-(4-(methylthio)phenyl)-3-oxo-1-propen-1-yl)phenoxy)-2-methyl-
16. Unii-2j3h5c81a5
17. Gft-505;elafibranor
18. Surecn815512
19. Elafibranor [who-dd]
20. Schembl815512
21. Chembl3707395
22. Schembl16552997
23. Gtpl11135
24. Ex-a757
25. Dtxsid601045330
26. Bcp19067
27. Zhb93288
28. Bdbm50502541
29. Mfcd27987940
30. S3720
31. Zinc114643710
32. Ccg-268462
33. Cs-5522
34. Db05187
35. (e)-2-(2,6-dimethyl-4-(3-(4-(methylthio)phenyl)-3-oxoprop-1-enyl)phenoxy)-2-methylpropanoic Acid
36. Ac-31455
37. Ac-35189
38. As-57112
39. Be163306
40. Hy-16737
41. D11208
42. A856857
43. J-690356
44. Q15409440
45. 2-(2,6-dimethyl-4-(3-(4-(methylsulfanyl)phenyl)-3-oxoprop- 1-en-1-yl)phenoxy)-2-methylpropanoic Acid
46. 2-(2,6-dimethyl-4-(3-(4-(methylthio)phenyl)-3-oxoprop-1-en-1-yl)phenoxy)-2-methylpropanoicacid
47. 2-[2,6-dimethyl-4-[(1e)-3-[4-(methylthio)phenyl]-3-oxo-1-propen-1-yl]phenoxy]-2-methylpropanoic Acid
48. Propanoic Acid, 2-(2,6-dimethyl-4-((1e)-3-(4-(methylthio)phenyl)-3-oxo-1-propen-1-yl)phenoxy)-
49. Propanoic Acid, 2-[2,6-dimethyl-4-[(1e)-3-[4-(methylthio)phenyl]-3-oxo-1-propen-1-yl]phenoxy]-2-methyl-
| Molecular Weight | 384.5 g/mol |
|---|---|
| Molecular Formula | C22H24O4S |
| XLogP3 | 5.1 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 7 |
| Exact Mass | 384.13953042 g/mol |
| Monoisotopic Mass | 384.13953042 g/mol |
| Topological Polar Surface Area | 88.9 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 537 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Investigated for use/treatment in atherosclerosis and diabetes mellitus type 2.
Treatment of primary biliary cholangitis
Treatment of non-alcoholic fatty liver disease (NAFLD) including non-alcoholic steatohepatitis (NASH)
GFT505 is an oral treatment that acts on the 3 sub-types of PPAR (PPARa, PPARg, PPARd) with a preferential action on PPARa. It has a sophisticated mechanism of action. It is able to differentially recruit cofactors to the nuclear receptor, which subsequently lead to differential regulation of genes and biological effect. Therefore, the ability to identify and profile the activity of selective nuclear receptor modulator (SNuRMs) is a powerful approach to select innovative drug candidates with improved efficacy and diminished side effects. These pluripotent and multimodal molecules have significant positive effects on obesity, insulin-resistance and diabetes, atherosclerosis, inflammation, and the lipid triad (increasing of HDL cholesterol, lowering of triglycerides and LDL cholesterol).
 Biophore is a research-driven global pharmaceutical company focused on niche APIs for the generic industry.
Biophore is a research-driven global pharmaceutical company focused on niche APIs for the generic industry.
 Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.
Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
 Biophore is a research-driven global pharmaceutical company focused on niche APIs for the generic industry.
Biophore is a research-driven global pharmaceutical company focused on niche APIs for the generic industry.
About the Company : Biophore, founded in 2007, develops and manufactures niche and complex pharmaceutical products. With USFDA- and EU-approved API facilities, a dedicated intermediates site and an R&...
 Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.
Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.
About the Company : Established in 2004, Metrochem API is one of the fastest-growing APIs, pellets & intermediates manufacturers. It has 6 dedicated manufacturing facilities for its 3 core product gro...
About the Company : HRV Pharma is a global manufacturer, seller, and exporter of APIs, intermediates, pellets, food-grade chemicals, food additives, and food ingredients. The company provides sourcing...
About the Company : Maithri Drugs Pvt. Ltd. is a global supplier of Active Pharmaceutical Ingredients (APIs), serving pharmaceutical companies in 60+ countries. Its API portfolio spans antivirals, ant...
About the Company : Enaltec, founded in 2006, specializes in developing and manufacturing complex, small-volume, technology-driven APIs at competitive prices, leveraging the India advantage for global...
About the Company : ApiSyn Healthcare, established in 2017, is a vertically integrated manufacturer of APIs & intermediates serving the global pharmaceutical industry. As part of SynZeal Research, a p...
About the Company : Cdymax (India) Pharma Private Limited was founded in 1994. The company's line of business includes the manufacturing, fabricating, or processing of drugs in pharmaceutical preparat...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Under the licensing agreement, Ipsen’s Iqirvo (elafibranor) has been granted pricing and reimbursement in Italy for Primary Biliary Cholangitis.
Lead Product(s): Elafibranor,Ursodeoxycholic Acid
Therapeutic Area: Hepatology (Liver, Pancreatic, Gall Bladder) Brand Name: Iqirvo
Study Phase: Approved FDFProduct Type: Miscellaneous
Sponsor: Ipsen
Deal Size: $542.8 million Upfront Cash: $135.7 million
Deal Type: Licensing Agreement May 20, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Elafibranor,Ursodeoxycholic Acid
Therapeutic Area : Hepatology (Liver, Pancreatic, Gall Bladder)
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Ipsen
Deal Size : $542.8 million
Deal Type : Licensing Agreement
GENFIT to Receive €26.5M Milestone After Ipsen’s Iqirvo® Pricing Approval in Italy
Details : Under the licensing agreement, Ipsen’s Iqirvo (elafibranor) has been granted pricing and reimbursement in Italy for Primary Biliary Cholangitis.
Product Name : Iqirvo
Product Type : Miscellaneous
Upfront Cash : $135.7 million
May 20, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Elafibranor is a Other Small Molecule drug candidate, which is currently being evaluated in phase III clinical studies for the treatment of Liver Cirrhosis, Biliary.
Lead Product(s): Elafibranor,Inapplicable
Therapeutic Area: Hepatology (Liver, Pancreatic, Gall Bladder) Brand Name: Undisclosed
Study Phase: Phase IIIProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable December 12, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Elafibranor,Inapplicable
Therapeutic Area : Hepatology (Liver, Pancreatic, Gall Bladder)
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
A Study of Elafibranor in Adult Japanese Participants With Primary Biliary Cholangitis (PBC)
Details : Elafibranor is a Other Small Molecule drug candidate, which is currently being evaluated in phase III clinical studies for the treatment of Liver Cirrhosis, Biliary.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
December 12, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Iqirvo (elafibranor) is an approved, novel, oral, PPAR alpha/delta agonist, as a treatment for patients with primary biliary cholangitis.
Lead Product(s): Elafibranor,Inapplicable
Therapeutic Area: Hepatology (Liver, Pancreatic, Gall Bladder) Brand Name: Iqirvo
Study Phase: Approved FDFProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable October 10, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Elafibranor,Inapplicable
Therapeutic Area : Hepatology (Liver, Pancreatic, Gall Bladder)
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
UK MHRA Approves Ipsen’s Elafibranor For Rare Liver Disease Treatment
Details : Iqirvo (elafibranor) is an approved, novel, oral, PPAR alpha/delta agonist, as a treatment for patients with primary biliary cholangitis.
Product Name : Iqirvo
Product Type : Miscellaneous
Upfront Cash : Inapplicable
October 10, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Iqirvo (elafibranor) is an approved, novel, oral, PPAR alpha/delta agonist, as a treatment for patients with primary biliary cholangitis.
Lead Product(s): Elafibranor,Inapplicable
Therapeutic Area: Hepatology (Liver, Pancreatic, Gall Bladder) Brand Name: Iqirvo
Study Phase: Approved FDFProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable September 20, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Elafibranor,Inapplicable
Therapeutic Area : Hepatology (Liver, Pancreatic, Gall Bladder)
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Ipsen’s Iqirvo® Approved in EU For Primary Biliary Cholangitis Treatment
Details : Iqirvo (elafibranor) is an approved, novel, oral, PPAR alpha/delta agonist, as a treatment for patients with primary biliary cholangitis.
Product Name : Iqirvo
Product Type : Miscellaneous
Upfront Cash : Inapplicable
September 20, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Iqirvo (elafibranor) is a novel, oral, dual peroxisome activated receptor (PPAR) alpha/delta agonist, recommended for the treatment of patients with primary biliary cholangitis.
Lead Product(s): Elafibranor,Inapplicable
Therapeutic Area: Hepatology (Liver, Pancreatic, Gall Bladder) Brand Name: Iqirvo
Study Phase: Approved FDFProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable July 26, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Elafibranor,Inapplicable
Therapeutic Area : Hepatology (Liver, Pancreatic, Gall Bladder)
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Ipsen Receives CHMP Opinions for Iqirvo® and Kayfanda® in Rare Cholestatic Liver Diseases
Details : Iqirvo (elafibranor) is a novel, oral, dual peroxisome activated receptor (PPAR) alpha/delta agonist, recommended for the treatment of patients with primary biliary cholangitis.
Product Name : Iqirvo
Product Type : Miscellaneous
Upfront Cash : Inapplicable
July 26, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Iqirvo (elafibranor) is a novel, oral, dual peroxisome activated receptor (PPAR) alpha/delta agonist, currently under investigation as a treatment for patients with primary biliary cholangitis.
Lead Product(s): Elafibranor,Inapplicable
Therapeutic Area: Hepatology (Liver, Pancreatic, Gall Bladder) Brand Name: Iqirvo
Study Phase: Approved FDFProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable June 10, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Elafibranor,Inapplicable
Therapeutic Area : Hepatology (Liver, Pancreatic, Gall Bladder)
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Ipsen's Iqirvo® Gets FDA Approval for Biliary Cholangitis
Details : Iqirvo (elafibranor) is a novel, oral, dual peroxisome activated receptor (PPAR) alpha/delta agonist, currently under investigation as a treatment for patients with primary biliary cholangitis.
Product Name : Iqirvo
Product Type : Miscellaneous
Upfront Cash : Inapplicable
June 10, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Elafibranor is a Other Small Molecule drug candidate, which is currently being evaluated in phase III clinical studies for the treatment of Liver Cirrhosis, Biliary.
Lead Product(s): Elafibranor,Inapplicable
Therapeutic Area: Hepatology (Liver, Pancreatic, Gall Bladder) Brand Name: Undisclosed
Study Phase: Phase IIIProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable April 25, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Elafibranor,Inapplicable
Therapeutic Area : Hepatology (Liver, Pancreatic, Gall Bladder)
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : Elafibranor is a Other Small Molecule drug candidate, which is currently being evaluated in phase III clinical studies for the treatment of Liver Cirrhosis, Biliary.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
April 25, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
GFT505 (elafibranor) is a novel, oral, once-daily, dual peroxisome activated receptor (PPAR) alpha/delta agonist, currently under investigation as a treatment for patients with primary biliary cholangitis.
Lead Product(s): Elafibranor,Inapplicable
Therapeutic Area: Hepatology (Liver, Pancreatic, Gall Bladder) Brand Name: Iqirvo
Study Phase: Phase IIIProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable December 07, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Elafibranor,Inapplicable
Therapeutic Area : Hepatology (Liver, Pancreatic, Gall Bladder)
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : GFT505 (elafibranor) is a novel, oral, once-daily, dual peroxisome activated receptor (PPAR) alpha/delta agonist, currently under investigation as a treatment for patients with primary biliary cholangitis.
Product Name : Iqirvo
Product Type : Miscellaneous
Upfront Cash : Inapplicable
December 07, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Elafibranor is a Other Small Molecule drug candidate, which is currently being evaluated in phase III clinical studies for the treatment of Liver Cirrhosis, Biliary.
Lead Product(s): Elafibranor,Inapplicable
Therapeutic Area: Hepatology (Liver, Pancreatic, Gall Bladder) Brand Name: Undisclosed
Study Phase: Phase IIIProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable August 30, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Elafibranor,Inapplicable
Therapeutic Area : Hepatology (Liver, Pancreatic, Gall Bladder)
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
A Long-Term Study of Elafibranor in Adult Participants With Primary Biliary Cholangitis
Details : Elafibranor is a Other Small Molecule drug candidate, which is currently being evaluated in phase III clinical studies for the treatment of Liver Cirrhosis, Biliary.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
August 30, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
GFT505 (elafibranor) is a novel, oral, once-daily, dual peroxisome activated receptor (PPAR) alpha/delta (α,δ) agonist, currently under investigation as a treatment for patients with PBC, a rare liver disease.
Lead Product(s): Elafibranor,Inapplicable
Therapeutic Area: Hepatology (Liver, Pancreatic, Gall Bladder) Brand Name: Iqirvo
Study Phase: Phase IIIProduct Type: Miscellaneous
Sponsor: Genfit
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable June 30, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Elafibranor,Inapplicable
Therapeutic Area : Hepatology (Liver, Pancreatic, Gall Bladder)
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Genfit
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : GFT505 (elafibranor) is a novel, oral, once-daily, dual peroxisome activated receptor (PPAR) alpha/delta (α,δ) agonist, currently under investigation as a treatment for patients with PBC, a rare liver disease.
Product Name : Iqirvo
Product Type : Miscellaneous
Upfront Cash : Inapplicable
June 30, 2023

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Global Sales Information

Patents & EXCLUSIVITIES
Patent Expiration Date : 2037-03-30
US Patent Number : 11850223
Drug Substance Claim :
Drug Product Claim :
Application Number : 218860
Patent Use Code : U-1854
Delist Requested :
Patent Use Description : TREATMENT OF PRIMARY B...
Patent Expiration Date : 2037-03-30

Patent Expiration Date : 2037-03-30
US Patent Number : 11331292
Drug Substance Claim :
Drug Product Claim :
Application Number : 218860
Patent Use Code : U-1854
Delist Requested :
Patent Use Description : TREATMENT OF PRIMARY B...
Patent Expiration Date : 2037-03-30

Patent Expiration Date : 2037-03-30
US Patent Number : 12310935
Drug Substance Claim :
Drug Product Claim :
Application Number : 218860
Patent Use Code : U-1854
Delist Requested :
Patent Use Description : TREATMENT OF PRIMARY B...
Patent Expiration Date : 2037-03-30

Patent Expiration Date : 2037-03-30
US Patent Number : 12295928
Drug Substance Claim :
Drug Product Claim :
Application Number : 218860
Patent Use Code : U-1854
Delist Requested :
Patent Use Description : TREATMENT OF PRIMARY B...
Patent Expiration Date : 2037-03-30

Patent Expiration Date : 2037-03-30
US Patent Number : 12295927
Drug Substance Claim :
Drug Product Claim :
Application Number : 218860
Patent Use Code : U-1854
Delist Requested :
Patent Use Description : TREATMENT OF PRIMARY B...
Patent Expiration Date : 2037-03-30

Patent Expiration Date : 2037-03-30
US Patent Number : 12233038
Drug Substance Claim :
Drug Product Claim :
Application Number : 218860
Patent Use Code : U-1854
Delist Requested :
Patent Use Description : TREATMENT OF PRIMARY B...
Patent Expiration Date : 2037-03-30

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Exclusivity Code : NCE
Exclusivity Expiration Date : 2029-06-10
Application Number : 218860
Product Number : 1
Exclusivity Details :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Exclusivity Code : ODE-486
Exclusivity Expiration Date : 2031-06-10
Application Number : 218860
Product Number : 1
Exclusivity Details :

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]ABOUT THIS PAGE
41
PharmaCompass offers a list of Elafibranor API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Elafibranor manufacturer or Elafibranor supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Elafibranor manufacturer or Elafibranor supplier.
PharmaCompass also assists you with knowing the Elafibranor API Price utilized in the formulation of products. Elafibranor API Price is not always fixed or binding as the Elafibranor Price is obtained through a variety of data sources. The Elafibranor Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Elafibranor manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Elafibranor, including repackagers and relabelers. The FDA regulates Elafibranor manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Elafibranor API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Elafibranor manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Elafibranor supplier is an individual or a company that provides Elafibranor active pharmaceutical ingredient (API) or Elafibranor finished formulations upon request. The Elafibranor suppliers may include Elafibranor API manufacturers, exporters, distributors and traders.
click here to find a list of Elafibranor suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
Elafibranor Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Elafibranor GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Elafibranor GMP manufacturer or Elafibranor GMP API supplier for your needs.
A Elafibranor CoA (Certificate of Analysis) is a formal document that attests to Elafibranor's compliance with Elafibranor specifications and serves as a tool for batch-level quality control.
Elafibranor CoA mostly includes findings from lab analyses of a specific batch. For each Elafibranor CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Elafibranor may be tested according to a variety of international standards, such as European Pharmacopoeia (Elafibranor EP), Elafibranor JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Elafibranor USP).

